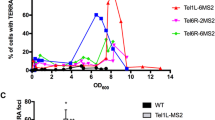
Abstract
The experimental evidence from the last decade made telomerase a prominent member of a family of moonlighting proteins performing different functions at various cellular loci. However, the study of extratelomeric functions of the catalytic subunit of mammalian telomerase (TERT) is often complicated by the fact that it is sometimes difficult to distinguish them from its role(s) at the chromosomal ends. Here, we present an experimental model for studying the extranuclear function(s) of mammalian telomerase in the yeast Saccharomyces cerevisiae. We demonstrate that the catalytic subunit of mammalian telomerase protects the yeast cells against oxidative stress and affects the stability of the mitochondrial genome. The advantage of using S. cerevisiae to study of mammalian telomerase is that (1) mammalian TERT does not interfere with its yeast counterpart in the maintenance of telomeres, (2) yeast telomerase is not localized in mitochondria and (3) it does not seem to be involved in the protection of cells against oxidative stress and stabilization of mtDNA. Thus, yeast cells can be used as a ‘test tube’ for reconstitution of mammalian TERT extranuclear function(s).




Similar content being viewed by others
References
Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, Birch-Machin MA, von Zglinicki T, Saretzki G (2008) Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J Cell Sci 121:1046–1053
Ale-Agha N, Dyballa-Rukes N, Jakob S, Altschmied J, Haendeler J (2014) Cellular functions of the dual-targeted catalytic subunit of telomerase, telomerase reverse transcriptase–potential role in senescence and aging. Exp Gerontol 56:189–193
Bah A, Bachand F, Clair E, Autexier C, Wellinger RJ (2004) Humanized telomeres and an attempt to express a functional human telomerase in yeast. Nucleic Acids Res 32:1917–1927
Blackburn EH (2010) Telomeres and telomerase: the means to the end (Nobel lecture). Angew Chem Int Ed Engl 49:7405–7421
Blaisonneau J, Nosek J, Fukuhara H (1999) Linear DNA plasmid pPK2 of Pichia kluyveri: distinction between cytoplasmic and mitochondrial linear plasmids in yeasts. Yeast 15:781–791
Bollmann FM (2008) The many faces of telomerase: emerging extratelomeric effects. Bioessays 30:728–732
Buchner N, Zschauer TC, Lukosz M, Altschmied J, Haendeler J (2010) Downregulation of mitochondrial telomerase reverse transcriptase induced by H2O2 is Src kinase dependent. Exp Gerontol 45:558–562
Chiodi I, Mondello C (2012) Telomere-independent functions of telomerase in nuclei, cytoplasm, and mitochondria. Front Oncol 2:133
Choi J, Southworth LK, Sarin KY, Venteicher AS, Ma W, Chang W, Cheung P, Jun S, Artandi MK, Shah N, Kim SK, Artandi SE (2008) TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet 4:e10
Chung HK, Cheong C, Song J, Lee HW (2005) Extratelomeric functions of telomerase. Curr Mol Med 5:233–241
Criddle DN, Gillies S, Baumgartner-Wilson HK, Jaffar M, Chinje EC, Passmore S, Chvanov M, Barrow S, Gerasimenko OV, Tepikin AV, Sutton R, Petersen OH (2006) Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J Biol Chem 281:40485–40492
Ding D, Zhou J, Wang M, Cong YS (2013) Implications of telomere-independent activities of telomerase reverse transcriptase in human cancer. FEBS J 280:3205–3211
Flores I, Evan G, Blasco MA (2006) Genetic analysis of myc and telomerase interactions in vivo. Mol Cell Biol 26:6130–6138
Galligan JT, Marchetti SE, Kennell JC (2011) Reverse transcription of the pFOXC mitochondrial retroplasmids of Fusarium oxysporum is protein primed. Mob DNA 2:1
Gietz RD, Schiestl RH, Willems AR, Woods RA (1995) Studies on the transformation of intact yeast cells by the LiAc/ssDNA/PEG procedure. Yeast 11:355–360
Gordon DM, Santos JH (2010) The emerging role of telomerase reverse transcriptase in mitochondrial DNA metabolism. J Nucleic Acids
Green MR, Sambrook J (2012) Molecular cloning: a laboratory manual, 4th edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Greenberg RA, O’Hagan RC, Deng H, Xiao Q, Hann SR, Adams RR, Lichtsteiner S, Chin L, Morin GB, DePinho RA (1999) Telomerase reverse transcriptase gene is a direct target of c-Myc but is not functionally equivalent in cellular transformation. Oncogene 18:1219–1226
Haendeler J, Hoffmann J, Brandes RP, Zeiher AM, Dimmeler S (2003) Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phosphorylation of tyrosine 707. Mol Cell Biol 23:4598–4610
Haendeler J, Hoffmann J, Diehl JF, Vasa M, Spyridopoulos I, Zeiher AM, Dimmeler S (2004) Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ Res 94:768–775
Haendeler J, Drose S, Buchner N, Jakob S, Altschmied J, Goy C, Spyridopoulos I, Zeiher AM, Brandt U, Dimmeler S (2009) Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler Thromb Vasc Biol 29:929–935
Hassan HM, Fridovich I (1979) Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys 196:385–395
Huberts DH, van der Klei IJ (2010) Moonlighting proteins: an intriguing mode of multitasking. Biochim Biophys Acta 1803:520–525
Iannilli F, Zalfa F, Gartner A, Bagni C, Dotti CG (2013) Cytoplasmic TERT associates to RNA granules in fully mature neurons: role in the translational control of the cell cycle inhibitor p15INK4B. PLoS One 8:e66602
Indran IR, Hande MP, Pervaiz S (2011) hTERT overexpression alleviates intracellular ROS production, improves mitochondrial function, and inhibits ROS-mediated apoptosis in cancer cells. Cancer Res 71:266–276
Jaiswal RK, Kumar P, Yadava PK (2013) Telomerase and its extracurricular activities. Cell Mol Biol Lett 18:538–554
Kinoshita T, Nagamatsu G, Saito S, Takubo K, Horimoto K, Suda T (2014) Telomerase reverse transcriptase has an extratelomeric function in somatic cell reprogramming. J Biol Chem 289:15776–15787
Koeppel F, Riou JF, Laoui A, Mailliet P, Arimondo PB, Labit D, Petitgenet O, Hélène C, Mergny JL (2001) Ethidium derivatives bind to G-quartets, inhibit telomerase and act as fluorescent probes for quadruplexes. Nucleic Acids Res 29:1087–1096
Kovalenko OA, Caron MJ, Ulema P, Medrano C, Thomas AP, Kimura M, Bonini MG, Herbig U, Santos JH (2010) A mutant telomerase defective in nuclear-cytoplasmic shuttling fails to immortalize cells and is associated with mitochondrial dysfunction. Aging Cell 9:203–219
Lambowitz A, Zimmerly S (2011) Group II introns: mobile ribozymes that invade DNA. Col Spring Harb Perspect Biol 3:a003616
Li Y, Tergaonkar V (2014) Noncanonical functions of telomerase: implications in telomerase-targeted cancer therapies. Cancer Res 74:1639–1644
Ling X, Wen L, Zhou Y (2012) Role of mitochondrial translocation of telomerase in hepatocellular carcinoma cells with multidrug resistance. Int J Med Sci 9:545–554
Listerman I, Sun J, Gazzaniga FS, Lukas JL, Blackburn EH (2013) The major reverse transcriptase-incompetent splice variant of the human telomerase protein inhibits telomerase activity but protects from apoptosis. Cancer Res 73:2817–2828
Livak K (1997) ABI Prism 7700 sequence detection system. User Bulletin 2. PE Applied Biosystems, Foster City, CA
Maida Y, Yasukawa M, Furuuchi M, Lassmann T, Possemato R, Okamoto N, Kasim V, Hayashizaki Y, Hahn WC, Masutomi K (2009) An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature 461:230–235
Maida Y, Yasukawa M, Okamoto N, Ohka S, Kinoshita K, Totoki Y, Ito TK, Minamino T, Nakamura H, Yamaguchi S, Shibata T, Masutomi K (2014) Involvement of telomerase reverse transcriptase in heterochromatin maintenance. Mol Cell Biol 34:1576–1593
Majerska J, Sykorova E, Fajkus J (2011) Non-telomeric activities of telomerase. Mol Biosyst 7:1013–1023
Masutomi K, Possemato R, Wong JM, Currier JL, Tothova Z, Manola JB, Ganesan S, Lansdorp PM, Collins K, Hahn WC (2005) The telomerase reverse transcriptase regulates chromatin state and DNA damage responses. Proc Natl Acad Sci USA 102:8222–8227
Mukherjee S, Firpo EJ, Wang Y, Roberts JM (2011) Separation of telomerase functions by reverse genetics. Proc Natl Acad Sci USA 108:E1363–E1371
Niedenthal RK, Riles L, Johnston M, Hegemann JH (1996) Green fluorescent protein as a marker for gene expression and subcellular localization in budding yeast. Yeast 12:773–786
Nozawa K, Maehara K, Isobe K (2001) Mechanism for the reduction of telomerase expression during muscle cell differentiation. J Biol Chem 276:22016–22023
Ogur M, John St R, Nagai S (1957) Tetrazolium overlay technique for population studies of respiration deficiency in yeast. Science 125:928–929
Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, McLaughlin M, Veenstra TD, Nusse R, McCrea PD, Artandi SE (2009) Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460:66–72
Perrault SD, Hornsby PJ, Betts DH (2005) Global gene expression response to telomerase in bovine adrenocortical cells. Biochem Biophys Res Commun 335:925–936
Polcic P, Forte M (2003) Response of yeast to the regulated expression of proteins in the Bcl-2 family. Biochem J 374:393–402
Rahman R, Latonen L, Wiman KG (2005) hTERT antagonizes p53-induced apoptosis independently of telomerase activity. Oncogene 24:1320–1327
Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA (2011) Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470:359–365
Santos JH, Meyer JN, Skorvaga M, Annab LA, Van Houten B (2004) Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell 3:399–411
Santos JH, Meyer JN, Van Houten B (2006) Mitochondrial localization of telomerase as a determinant for hydrogen peroxide-induced mitochondrial DNA damage and apoptosis. Hum Mol Genet 15:1757–1768
Saretzki G (2009) Telomerase, mitochondria and oxidative stress. Exp Gerontol 44:485–492
Saretzki G (2014) Extra-telomeric functions of human telomerase: cancer, mitochondria and oxidative stress. Curr Pharm Des. doi:10.2174/1381612820666140630095606
Seimiya H, Sawada H, Muramatsu Y, Shimizu M, Ohko K, Yamane K, Tsuruo T (2000) Involvement of 14-3-3 proteins in nuclear localization of telomerase. EMBO J 19:2652–2661
Sharma NK, Reyes A, Green P, Caron MJ, Bonini MG, Gordon DM, Holt IJ, Santos JH (2012) Human telomerase acts as a hTR-independent reverse transcriptase in mitochondria. Nucleic Acids Res 40:712–725
Shin KH, Kang MK, Dicterow E, Kameta A, Baluda MA, Park NH (2004) Introduction of human telomerase reverse transcriptase to normal human fibroblasts enhances DNA repair capacity. Clin Cancer Res 10:2551–2560
Shkreli M, Sarin KY, Pech MF, Papeta N, Chang W, Brockman SA, Cheung P, Lee E, Kuhnert F, Olson JL, Kuo CJ, Gharavi AG, D’Agati VD, Artandi SE (2012) Reversible cell-cycle entry in adult kidney podocytes through regulated control of telomerase and Wnt signaling. Nat Med 18:111–119
Sickmann A, Reinders J, Wagner Y, Joppich C, Zahedi R, Meyer HE, Schonfisch B, Perschil I, Chacinska A, Guiard B, Rehling P, Pfanner N, Meisinger C (2003) The proteome of Saccharomyces cerevisiae mitochondria. Proc Natl Acad Sci USA 100:13207–13212
Singhapol C, Pal D, Czapiewski R, Porika M, Nelson G, Saretzki GC (2013) Mitochondrial telomerase protects cancer cells from nuclear DNA damage and apoptosis. PLoS One 8:e52989
Smith LL, Coller HA, Roberts JM (2003) Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat Cell Biol 5:474–479
Storici F, Bebenek K, Kunkel TA, Gordenin DA, Resnick MA (2007) RNA-templated DNA repair. Nature 447:338–341
Sung YH, Ali M, Lee HW (2014) Extracting extra-telomeric phenotypes from telomerase mouse models. Yonsei Med J 55:1–8
Taylor SD, Zhang H, Eaton JS, Rodeheffer MS, Lebedeva MA, O’Rourke TW, Siede W, Shadel GS (2005) The conserved Mec1/Rad53 nuclear checkpoint pathway regulates mitochondrial DNA copy number in Saccharomyces cerevisiae. Mol Cell Biol 16:3010–3018
Walther TC, Kennell JC (1999) Linear mitochondrial plasmids of F. oxysporum are novel, telomere-like retroelements. Mol Cell 4:229–238
Wege H, Heim D, Lutgehetmann M, Dierlamm J, Lohse AW, Brummendorf TH (2011) Forced activation of beta-catenin signaling supports the transformation of hTERT-immortalized human fetal hepatocytes. Mol Cancer Res 9:1222–1231
Westermann B, Neupert W (2000) Mitochondria-targeted green fluorescent proteins: convenient tools for the study of organelle biogenesis in Saccharomyces cerevisiae. Yeast 16:1421–1427
Xiang H, Wang J, Mao Y, Liu M, Reddy VN, Li DW (2002) Human telomerase accelerates growth of lens epithelial cells through regulation of the genes mediating RB/E2F pathway. Oncogene 21:3784–3791
Yang C, Przyborski S, Cooke MJ, Zhang X, Stewart R, Anyfantis G, Atkinson SP, Saretzki G, Armstrong L, Lako M (2008) A key role for telomerase reverse transcriptase unit in modulating human embryonic stem cell proliferation, cell cycle dynamics, and in vitro differentiation. Stem Cells 26:850–863
Acknowledgments
We thank Ladislav Kovac for inspiration and continuous support, H. Yde Steensma (Leiden University, The Netherlands) for the S. cerevisiae strain GG595, Johannes H. Hegemann (Heinrich-Heine-Universität, Düsseldorf, Germany) for the plasmid pUG35-yEGFP3, Ronald A. DePinho (University of Texas, USA) for the plasmid pGRN188, Juraj Kramara (Palacky University in Olomouc, Czech republic) for technical assistance and Jiri Bartek (Palacky University in Olomouc, Czech republic) for providing the possibility to perform fluorescent microscopy in his laboratory. We also thank two anonymous reviewers for their constructive comments, which helped us to improve the manuscript. This work was supported in part by the Slovak grant agencies APVV (0035-11 and 0123-10), VEGA (1/0311/12) and Comenius University (UK/409/2014) and National Institutes of Health Grants 2R01ES013773-06A1.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Hohmann.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Simonicova, L., Dudekova, H., Ferenc, J. et al. Saccharomyces cerevisiae as a model for the study of extranuclear functions of mammalian telomerase. Curr Genet 61, 517–527 (2015). https://doi.org/10.1007/s00294-014-0472-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-014-0472-8