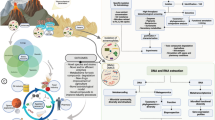
Abstract
This work focuses on rock-inhabiting fungi (RIF) of Antarctic rocky deserts, considered the closest to a possible Martian habitat, as the best example of adaptation to the extremes. The study of RIF ecophysiology, resistance and adaptation provides tools that shed light on the evolution of extremophily. These studies also help define the actual limits for life and provide insight for investigating its existence beyond our planet. The scientific results obtained from over 20 years of research on the biodiversity, phylogeny and evolution toward extremotolerance reviewed here demonstrate how these fascinating organisms can withstand conditions well beyond those in their natural environment. A final focus is given on results and perspectives arising from a recent proteomic approach, and from astrobiological experiments and their significance for future space exploration. These studies demonstrate that Antarctic RIF offer an excellent opportunity to investigate many basic, but also applicative areas of research on extremophily.





Similar content being viewed by others
References
Åkerfelt M, Morimoto RI, Sistonen L (2010) Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol 11:545–555
Andersen DT, McKay CP, Wharton RA, Rummel JD (1992) Testing a Mars science outpost in the Antarctic dry valleys. Adv Space Res 12:205–209
Askin RA (1992) Late Cretaceous–early Tertiary Antarctic outcrop evidence for past vegetation and climates. The Antarctic Paleoenvironment: a perspective on global change: Part One 61–74
Baker BJ, Lutz MA, Dawson SC, Bond PL, Banfield JF (2004) Metabolically active eukaryotic communities in extremely acidic mine drainage. Appl Environ Microbiol 70:6264–6271
COSPAR (2011) COSPAR Planetary Protection Policy (20 Oct 2002, as amended 24 Mar 2011), COSPAR, Paris. Available online at http://cosparhq.cnes.fr/Scistr/PPPolicy%20%2824-Mar2011%29.pdf
Dadachova E, Casadevall A (2008) Ionizing radiation: how fungi cope, adapt, and exploit with the help of melanin. Curr Opin Microbiol 11:525–531
Dadachova E, Bryan RA, Huang X, Moadel T, Schweizer AD, Aisen P, Nosanchuk JD, Casadevall A (2007) Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS One 5:1–13
de los Ríos A, Wierzchos J, Ascaso C (2014) The lithic microbial ecosystems of Antarctica’s McMurdo Dry Valleys. Antarct Sci 26:459–477
de los Ríos A, Wierzchos J, Sancho LG, Ascaso C (2003) Acid microenvironments in microbial biofilms of Antarctic endolithic microecosystems. Eviron Microbiol 5:231–237
de Vera JP, Böttger U, de la Torre Noetzel R, Sánchez FJ, Grunow D, Schmitz N, Lange C, Hübers HW, Billi D, Baqué M, Rettberg P, Rabbow E, Reitz G, Berger T, Möller R, Bohmeier M, Horneck G, Westall F, Jänchen J, Fritz J, Meyer C, Onofri S, Selbmann L, Zucconi L, Kozyrovska N, Leya T, Foing B, Demets R, Cockell CS, Bryce C, Wagner D, Serrano P, Howell GME, Joshi J, Huwe B, Ehrenfreund P, Elsaesser A, Ott S, Meessen J, Feyh N, Szewzyk U, Jaumann R, Spohn T (2012) Supporting Mars exploration: BIOMEX in Low Earth Orbit and further astrobiological studies on the Moon using Raman and PanCam technology. Planet Space Sci 74:103–110
Doran PT, McKay CP, Clow GD, Dana GL (2002) Valley floor climate observations from the McMurdo Dry Valleys. Antarctica 1986–2000. Geophys Res 107:4772
Egidi E, de Hoog GS, Isola D, Onofri S, Quaedvlieg W, de Vries M, Verkley GJM, Stielow JB, Zucconi L, Selbmann L (2014) Phylogeny and taxonomy of meristematic rock-inhabiting black fungi in the Dothideomycetes based on multi-locus phylogenies. Fungal Divers 65:127–165
Fountain AG, Dana GL, Lewis KJ, Vaughn BH, McKnight DM (1998) Glaciers of the McMurdo Dry Valleys, Southern Victoria Land, Antarctica. In: Priscu JC (ed) Ecosystem Dynamics in a Polar Desert. American Geophysical Union, Antarctic Research Series Washington, D.C, pp 65–75
Fountain AG, Nylen TH, Monagh A, Basagic HJ, Bromwich D (2010) Snow in the McMurdo Dry Valleys, Antarctica. Int J Climatol 30:633–642
Francis JE, Poole I (2002) Cretaceous and early Tertiary climates of Antarctica: evidence from fossil wood. Palaeogeogr Palaeocl 182:47–64
Friedmann EI (1982) Endolithic microorganisms in the Antarctic cold desert. Science 215:1045–1053
Friedmann EI, Ocampo-Friedmann R (1976) Endolithic blue-green algae in the dry valleys: primary producers in the Antarctic desert ecosystem. Science 193:1247–1249
Friedmann EI, Hua M, Ocampo-Friedmann R (1988) Cryptoendolithic Lichen and Cyanobacterial Communities of the Ross Desert, Antarctica. Polarforschung 58:251–259
Gadd GL, de Rome L (1988) Biosorption of copper by fungal melanin. Appl Microbiol Biotechnol 29:610–617
Golubic S, Friedmann IE, Schneider J (1981) The lithobiontic ecological niche, with special reference to microorganisms. J Sediment Res 51:475–478
Gorbushina AA (2007) Life on the rocks. Environ Microbiol 9:1613–1631
Gorbushina AA, Whitehead K, Dornieden T, Niesse A, Schulte A, Hedges JI (2003) Black fungal colonies as units of survival: hyphal mycosporines synthesized by rock-dwelling micro-colonial fungi. Can J Bot 81:131–138
Gorbushina AA, Kotlova ER, Sherstneva OA (2008) Cellular responses of microcolonial rock fungi to long-term desiccation and subsequent rehydration. Stud Mycol 61:91–97
Gostinčar C, Grube M, Gunde-Cimerman N (2011) Evolution of fungal pathogens in domestic environments? Fungal Biol 115:1008–1018
Gueidan C, Ruibal C, de Hoog GS, Gorbushina AA, Untereiner WA, Lutzoni F (2008) A rock-inhabiting ancestor for mutualistic and pathogen-rich fungal lineages. Stud Mycol 61:111–119
Gueidan C, Ruibal C, de Hoog GS, Schneider H (2011) Rock-inhabiting fungi originated during periods of dry climate in the late Devonian and middle Triassic. Fungal Biol 115:987–996
Gunde-Cimerman N, Zalar P, de Hoog S, Plemenitaš A (2000) Hypersaline waters in salterns: natural ecological niches for halophilic black yeasts. FEMS Microbiol Ecol 32:235–240
Harutyunyan S, Muggia L, Grube M (2008) Black fungi in lichens from seasonally arid habitats. Stud Mycol 61:83–90
Hofmann GE, Buckley BA, Airaksinen S, Keen JE, Somero GN (2000) Heat-shock protein expression is absent in the Antarctic Fish Trematomus bernacchii (Family Nototheniidae). J Exp Biol 203:2331–2339
Isola D, Marzban G, Selbmann L, Onofri S, Laimer M, Sterflinger K (2011) Sample preparation and 2- DE procedure for protein expression profiling of black microclonial fungi. Fungal Biology 10:971–977. doi:10.1016/j.funbio.2011.03.001
Isola D, Selbmann L, de Hoog GS, Fenice M, Onofri S, Prenafeta-Boldú FX, Zucconi L (2013) Isolation and screening of black fungi as degraders of volatile aromatic hydrocarbons. Mycopathologia 175:369–379. doi:10.1007/s11046-013-9635-2
Kogej T, Wheeler MH, Lanisnik-Rižner T, Gunde-Cimermann N (2003) Inhibition of DHN-melanin biosynthesis by tricyclazole in Hortaea wernekii. In: Wolf K, Breunig G, Barth G (eds) Non conventional yeasts in genetics, biochemistry and biotechnology. Springer, Berlin Heidelberg New York, pp 143–148
Kogej T, Wheeler MH, Rizˇner TL, Gunde-Cimerman N (2004) Evidence for 1,8-dihydroxy-naphthalene melanin in three halophilic black yeasts grown under saline and non-saline conditions. FEMS Microbiol Lett 232:203–209
Kregel KC (2002) Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 92:2177–2186
Marzban G, Tesei D, Sterflinger K (2013) A Review beyond the borders: Proteomics of microclonial black fungi and black yeasts. Nat Sci 5:640–645. doi:10.4236/ns.2013.55079
Miller RW (2005) Viewpoint: Millennial Fever, Extremophiles, NASA, Astroenvironmentalism, and Planetary Protection. Electron Green J 1:1–9
Moeller R, Schuerger AC, Reitz G, Nicholson WL (2012) Protective role of spore structural components in determining Bacillus sublitis spore resistance to simulated Mars surface conditions. Appl Environ Microbiol 87:8849–8853
Moses V, Holm-Hansen O, Calvin M (1959) Non photosynthetic fixation of carbon dioxide by three microorganisms. J Bacteriol 77:70–78
Muller HJ (1964) The relation of recombination to mutational advantage. Mutat Res 1:2–9
Mustard JF, Murchie SL, Pelkey SM, Ehlmann BL, Milliken RE, Grant JA, Bibring J-P, Poulet F, Bishop J, Noe Dobrea E, Roach L, Seelos F, Arvidson RE, Wiseman S, Green R, Hash C, Humm D, Malaret E, McGovern JA, Seelos K, Clancy T, Clark R, Marais DD, Izenberg N, Knudson A, Langevin Y, Martin T, McGuire R, Morris M, Robinson T, Roush M, Smith G, Swayze P, Taylor H, Titus T, Wolff M (2008) Hydrated silicate minerals on Mars observed by the Mars reconnaissance orbiter CRISM instrument. Nature 454:305–309
Nienow JA, Friedmann EI (1993) Terrestrial lithophytic (rock) communities. In: Friedmann EI (ed) Antarctic microbiology. Wiley-Liss, New York, pp 343–412
Onofri S, Selbmann L, Zucconi L, Pagano S (2004) Antarctic microfungi as models for exobiology. Planet Space Sci 52:229–237
Onofri S, Zucconi L, Tosi S (2006) Continental Antarctic Fungi. IHW-Verlag, Eching
Onofri S, Zucconi L, Selbmann L, de Hoog GS, de los Rios A, Ruisi S, Grube M (2007a) Fungal Association at the cold edge of life. In: Seckbach J (ed) Algae and cyanobacteria in extreme environments; cellular origin, life in extreme habitats and astrobiology. Springer-Verlag, Berlin, p 450 (pp 739–757. ISSN: 1566–0400)
Onofri S, Selbmann L, de Hoog GS, Grube M, Barreca D, Ruisi S, Zucconi L (2007b) Evolution and adaptation of fungi at boundaries of life. Adv Space Res 40:1657–1664
Onofri S, Barreca D, Selbmann L, Isola D, Rabbow E, Horneck G, de Vera JPP, Hatton J, Zucconi L (2008) Resistance of Antarctic black fungi and cryptoendolithic communities to simulated space and Mars conditions. Stud Mycol 61:99–109
Onofri S, Selbmann L, Barreca D, Isola D, Zucconi L (2009) Do fungi survive under actual space conditions? Searching for evidence in favour of lithopanspermia. Plant Biosyst 143:S85–S87
Onofri S, de la Torre R, de Vera JP, Ott S, Zucconi L, Selbmann L, Scalzi G, Venkateswaran K, Rabbow E, Horneck G (2012) Survival of rock-colonizing organisms after 1.5 years in outer space. Astrobiology 12:508–516
Palmer RJ, Friedman EI (1988) Incorporation of inorganic carbon by Antarctic cryptoendolithic fungi. Polarforschung 58:189–191
Plemenitaš A, Vaupotič T, Lenassi M, Kogej T, Gunde-Cimerman N (2008) Adaptation of extremely halotolerant black yeast Hortaea werneckii to increased osmolarity: a molecular perspective at a glance. Stud Mycol 61:67–75
Quaedvlieg W, Binder M, Groenewald JZ, Summerell BA, Carnegie AJ, Burgess TI, Crous PW (2014) Introducing the Consolidated Species Concept to resolve species in the Teratosphaeriaceae. Persoonia 33:1–40
Rabbow E, Horneck G, Rettberg P, Schott J-U, Panitz C, L’Afflitto A, von Heise-Rotenburg R, Willnecker R, Baglioni P, Hatton J, Dettmann J, Demets R, Reitz G (2009) EXPOSE, an astrobiological exposure facility on the International Space Station—from proposal to flight. Origin Life Evol B 39:581–598
Rabbow E, Rettberg P, Barczyk S, Bohmeier M, Parpart A, Panitz C, Horneck G, von heise-rotenburg R, Hoppenbrouwers T, Willnecker R, Baglioni P, Demets R, Dettmann J, Reitz G (2012) EXPOSE-E: an ESA astrobiology mission 1.5 years in space. Astrobiology 12:374–386
Ruibal C (2004) Isolation and characterization of melanized, slow-growing fungi from semiarid rock surfaces of central Spain and Mallorca. PhD Dissertation, Universidad Autónoma de Madrid
Ruibal C, Gonzalo P, Bills GF (2005) Isolation and characterization of melanized fungi from limestone formations in Mallorca. Mycol Prog 4:23–38
Ruibal C, Gueidan C, Selbmann L, Gorbushina AA, Crous PW, Groenewald JZ, Muggia L, Grube M, Isola D, Schoch CL, Staley JT, Lutzoni F, de Hoog GS (2009) Phylogeny of rock inhabiting fungi related to Dothideomycetes. Stud Mycol 64:123–133
Ruisi S, Barreca D, Selbmann L, Zucconi L, Onofri S (2007) Fungi in Antarctica. Rev Environ Sci Biotech 6:127–141
Scalzi G, Selbmann L, Zucconi L, Rabbow E, Horneck G, Albertano P, Onofri S (2012) LIFE Experiment: Isolation of cryptoendolithic organisms from Antarctic colonized sandstone exposed to space and simulated Mars conditions on the International Space Station. Origin Life Evol B 42:253–262
Selbmann L, Onofri S, Fenice M, Federici F, Petruccioli M (2002) Production and structural characterisation of the exopolysaccharide of the Antarctic fungus Phoma herbarum CCFEE 5080. Res Microbiol 153:585–592
Selbmann L, de Hoog GS, Mazzaglia A, Friedmann EI, Onofri S (2005) Fungi at the edge of life: cryptoendolithic black fungi from Antarctic deserts. Stud Mycol 51:1–32
Selbmann L, de Hoog GS, Gerrits Van Den Ende AHG, Ruibal C, De Leo F, Zucconi L, Isola D, Ruisi S, Onofri S (2008) Drought meets acid: three new genera in a Dothidealean clade of extremotolerant fungi. Stud Mycol 61:1–20
Selbmann L, Zucconi L, Ruisi S, Grube M, Cardinale M, Onofri S (2010) Culturable bacteria associated with Antarctic lichens: affiliation and psychrotolerance. Polar Biol 33:71–83
Selbmann L, Isola D, Zucconi L, Onofri S (2011) Resistance to UV-B induced DNA damage in extreme-tolerant cryptoendolithic Antarctic fungi: detection by PCR assays. Fungal Biol 115:937–944
Selbmann L, Isola D, Fenice F, Zucconi L, Sterflinger K, Onofri S (2012) Potential extinction of Antarctic endemic fungal species as a consequence of Global Warming. Sci Tot Environ 438:127–134
Selbmann L, Egidi E, Isola D, Onofri S, Zucconi Z, de Hoog GS, Chinaglia S, Testa L, Tosi S, Balestrazzi A, Lantieri A, Compagno R, Tigini V, Varese G (2013a) Biodiversity, evolution and adaptation of fungi in extreme environments. Plant Biosyst 147:237–246
Selbmann L, Grube M, Onofri S, Isola D, Zucconi Z (2013b) Antarctic epilithic lichens as niches for black meristematic fungi. Biology 2:784–797. doi:10.3390/biology2020784
Selbmann L, Isola D, Egidi E, Zucconi L, Gueidan C, de Hoog GS, Onofri S (2014a) Mountain tips as reservoirs for new rock-fungal entities: Saxomyces gen. nov. and four new species from the Alps. Fungal Divers 65:167–182
Selbmann L, de Hoog GS, Zucconi L, Isola D, Onofri S (2014b) Black Yeasts From Cold Habitats. In: Buzzini P, Margesin R (eds) Yeasts From Cold Habitats. Springer-Verlag, Berlin, pp 173–189
Sert HB, Sümbül H, Sterflinger K (2007a) Microcolonial fungi from antique marbles in Perge/Side/Termessos (Antalya/Turkey). A Van Leeuw 91:217–227
Sert HB, Sümbül H, Sterflinger K (2007b) Sarcinomyces sideticae, a new black yeast from historical marble monuments in Side (Antalya, Turkey). Bot J Linn Soc 154:373–380
Sert HB, Sümbül H, Sterflinger K (2007c) A new species of Capnobotryella from monument surfaces. Mycol Res 111:1235–1241
Sterflinger K (1998) Temperature and NaCl-tolerance of rock inhabiting meristematic fungi. A van Leeuwenhoek 74:271–281
Sterflinger K (2006) Black yeasts and meristematic fungi: ecology, diversity and identification. In: Rosa C, Gabor P (eds) Yeast handbook: biodiversity and ecophysiology of yeasts. Springer, New York, pp 505–518
Sterflinger K, De Hoog GS, Haase G (1999) Phylogeny and ecology of meristematic ascomycetes. Stud Mycol 43:5–22
Sterflinger K, Tesei D, Zakharova K (2012) Fungi in hot and cold deserts with particular reference to micro- colonial fungi. Fungal Ecol 5:453–462. doi:10.1016/j.funeco.2011.12.007
Tesei D, Marzban G, Zakharova K, Isola D, Selbmann L, Sterflinger K (2012) Alteration of Protein Patterns in Black Rock Inhabiting Fungi as a Response to Different Temperatures. Fungal Biol 116:932–940
van Uden N (1984) Temperature profiles of yeasts. Adv Microbiol Physiol 25:195–251
Weinstein RN, Montiel PO, Johnstone K (2000) Influence of growth temperature on lipid and soluble carbohydrate synthesis by fungi isolated from fellfield soil in the maritime Antarctic. Mycologia 92:222–229
Wollenzien U, de Hoog GS, Krumbein WE, Urzì C (1995) On the isolation of microcolonial fungi occurring on and in marble and other calcareous rocks. Sci Tot Environ 167:287–294
Zakharova K, Tesei D, Marzban G, Dijksterhuis J, Wyatt T, Sterflinger K (2013) Microcolonial fungi on rocks: a life in constant drought? Mycopathologia 175:537–547
Zakharova K, Sterflinger K, Razzazi-Fazeli E, Noebauer K, Marzban G (2014a) Global proteomics of the extremophile black fungus Cryomyces antarcticus using 2D-Electrophoresis. Nat Sci 6:978–995
Zakharova K, Marzban G, de Vera JP, Lorek A, Sterflinger K (2014b) Protein patterns of black fungi under simulated Mars-like conditions. Sci Rep 4:5114
Zhdanova NN, Zakharchenko VA, Vember VV, Nakonechnaya LT (2000) Fungi from Chernobyl: mycobiota of the inner regions of the containment structures of the damaged nuclear reactor. Mycol Res 104:1421–1426
Zucconi L, Onofri S, Cecchini C, Isola D, Ripa C, Fenice M, Madonna S, Reboleiro-Rivas, Selbmann L (2014) Mapping the lithic colonization at the boundaries of life in Northern Victoria Land, Antarctica. Polar Biol (In Press)
Acknowledgments
The authors are grateful to Dr. Steven Emslie (University of North Carolina Wilmington) for accurate English revision of the text. This work is in the framework of the Italian National Program for Antarctic Researches (PNRA), at present funded by two projects (PROP09_68 and 2013/AZ1.17). The Italian National Antarctic Museum “Felice Ippolito” is kindly acknowledged for funding CCFEE (Culture Collection of Fungi From Extreme Environments). The ESA projects LIFE and BIOMEX and the ASI projects DC-MIC-2011-036 and DC-MIC-2011-036 are also acknowledged for supporting research in astrobiology. This review was supported by a grant from the São Paulo Research Foundation (FAPESP) of Brazil # 2014/01229-4.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. E. N. Rangel.
This article is part of the Special Issue “Fungal Stress Responses”.
Rights and permissions
About this article
Cite this article
Selbmann, L., Zucconi, L., Isola, D. et al. Rock black fungi: excellence in the extremes, from the Antarctic to space. Curr Genet 61, 335–345 (2015). https://doi.org/10.1007/s00294-014-0457-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-014-0457-7