Abstract
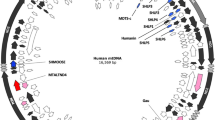
The mitochondrial (mt) genome of the sea anemone Metridium senile contains genes for only two transfer RNAs (tRNAs), tRNAf-Met and tRNATrp. Experiments were conducted to seek evidence for the occurrence of functional tRNAs corresponding to these genes and for the participation of nuclear DNA-encoded tRNAs in mt-protein synthesis. RNA sequences corresponding to the two mt-tRNA genes were located in mitochondria and it was shown that 3′-CC (and possibly A, but no other nucleotide) is added post-transcriptionally to the 3′ end of at least 50 % of mt-tRNAf-Met molecules and to a small fraction of the mt-tRNATrp molecules. Using specific oligonucleotide primers based on expected nuclear DNA-encoded tRNAs in a series of RACE experiments, we located the nuclear genes for tRNAGln, tRNAIle, tRNAi-Met, tRNAVal and tRNAThr. Data from Northern blot analyses indicated that mtDNA-encoded tRNAf-Met is limited to mitochondria but that nuclear DNA-encoded tRNAVal and tRNAi-Met are present in the cytoplasm and in mitochondria. These data provide direct evidence that in M. senile, mature, functional tRNAs are transcribed from the mtDNA-encoded tRNAf-Met and tRNATrp genes, and are consistent with the interpretation that both nuclear DNA-encoded tRNAVal and tRNAi-Met are utilized in mitochondrial and cytosolic protein synthesis.






Similar content being viewed by others
References
Abascal F, Posada D, Zardoya R (2012) The evolution of the mitochondrial genetic code in arthropods revisited. Mitochondr DNA 23:84–91
Adshya S (2008) Leishmania mitochondrial tRNA importers. Int J Biochem Cell Biol 40:2681–2685
Alfonzo JD, Soll D (2009) Mitochondrial tRNA import—the challenge to understand has just begun. Biol Chem 390:717–722
Anderson S, Bankier AT, Barrell BG, de Bruijn MHL, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJH, Staden R, Young IG (1981) Sequence and organization of the human mitochondrial genome. Nature 290:457–465
Barrell BG, Bankier AT, Drouin J (1979) A different genetic code in human mitochondria. Nature 282:189–194
Barrell BG, Anderson S, Bankier AT, de Bruijn MHL, Chen E, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJH, Staden R, Young IG (1980) Different pattern of codon recognition by mammalian mitochondrial tRNAs. Proc Natl Acad Sci USA 77:3164–3166
Beagley CT, Macfarlane JL, Pont-Kingdon GA, Okimoto R, Okada NA, Wolstenholme DR (1995) Mitochondrial genomes of Anthozoa (Cnidaria). In: Palmieri F, Papa P, Saccone C, Gadaleta N (eds) Progress in cell research: symposium on “30 years of progress in mitochondrial bioenergetics and molecular biology”. Elsevier Science BV, Amsterdam, pp 149–153
Beagley CT, Okada NA, Wolstenholme DR (1996) Two mitochondrial group I introns in a metazoan, the sea anemone Metridium senile: one intron contains genes for subunits 1 and 3 of NADH dehydrogenase. Proc Natl Acad Sci USA 93:5619–5623
Beagley CT, Okimoto R, Wolstenholme DR (1998) The mitochondrial genome of the sea anemone, Metridium senile (Cnidaria): introns, a paucity of tRNA genes, and a near standard genetic code. Genetics 148:1091–1108
Beagley CT, Okimoto R, Wolstenholme DR (1999) Mytilus mitochondrial DNA contains a functional gene for a tRNASer(UCN) with a dihydrouridine arm replacement loop, and a pseudo-tRNASer(UCN) gene. Genetics 152:641–652
Beaton MJ, Roger AJ, Calvalier-Smith T (1998) Sequence analysis of the mitochondrial genome of Sarcophyton glaucum: conserved gene order among octocorals. J Mol Evol 47:697–708
Bernt M, Braband A, Schierwater B, Peter F. Stadler PF (2012) Genetic aspects of mitochondrial genome evolution. Mol Phylogenet Evol, in press
Bibb MH, van Etten RA, Wright CT, Walberg MW, Clayton DA (1981) Sequence and gene organization of mouse mitochondrial DNA. Cell 26:167–180
Boore JL (1999) Animal mitochondrial genomes. Nucl Acids Res 27:1767–1780
Brown TA, Cecconi C, Tkachuk AN, Bustamante C, Clayton DA (2005) Replication of mitochondrial DNA occurs by strand displacement with alternative light-strand origins, not via a strand-coupled mechanism. Gene Dev 19:2466–2476
Burger G, Plante I, Lonergan KM, Gray MW (1995) The mitochondrial DNA of the amoeboid protozoon, Acanthamoeba castellanii: complete sequence, gene content and genome organization. J Mol Biol 245:522–537
Chomyn A, Hunkapillar MW, Attardi G (1981) Alignment of the amino terminal amino acid sequence of human cytochrome c oxidase subunits I and II with the sequence of their putative mRNAs. Nucl Acids Res 9:867–877
Clary DO, Wolstenholme DR (1985) The mitochondrial DNA molecule of Drosophila yakuba: nucleotide sequence, gene organization and genetic code. J Mol Evol 22:252–271
De Bruijn MHL, Schreier PH, Eperon IC, Barrell BG, Chen EY, Armstrong PW, Wong JFH, Roe BA (1980) A mammalian mitochondrial serine transfer RNA lacking the “dihydrouridine” loop and stem. Nucl Acids Res 8:5213–5522
Deutscher MP (1990) Ribonucleases, tRNA nucleotidyltransferase, and the 3′ processing of tRNA. Prog Nucl Acid Res Mol Biol 39:209–240
Dirheimer G, Keith G, Dumas P, Westhof E (1995) Primary, secondary and tertiary structures of tRNAs. In: Soll D, RajBhandary UL (eds) tRNA: structure, biosynthesis, and function. ASM press, Washington DC, pp 93–126
Don RH, Cox PT, Wainwright BJ, Baker K, Mattick JS (1991) Touchdown PCR to circumvent spurious priming during gene amplification. Nucl Acids Res 19:4008
Duchene AM, Pujol C, Mare′chal-Drouard L (2009) Import of tRNAs and aminoacyl-tRNA synthetases into mitochondria. Curr Genet 55:1–18
Esseiva AC, Marechal-Drouard L, Cosset A, Schneider A (2004) The t-stem determines the cytosolic or mitochondrial localization of Trypanosomal tRNAsMet. Mol Biol Cell 15:2750–2757
Frohman MA (1990) RACE: rapid amplification of cDNA ends. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols, a guide to methods and applications. Academic Press Inc, California, pp 28–38
Fukami H, Knowlton N (2005) Analysis of complete mitochondrial DNA sequences of three members of the coral species complex (Cnidaria, Anthozoa, Scleractinia). Coral Reefs 24:410–417
Gissi C, Iannelli F, Pesole G (2008) Evolution of the mitochondrial genome of metazoa as exemplified by comparison of congeneric species. Heredity 101:301–320
Haen KM, Pett W, Lavrov DV (2010) Parallel loss of nuclear-encoded mitochondrial aminoacyl-tRNA synthetases and mtDNA-encoded tRNAs in Cnidaria. Mol Biol Evol 27:2216–2219
Hancock K, Hajduk SL (1990) The mitochondrial tRNAs of Trypanosoma brucei are nuclear-encoded. J Biol Chem 265:19208–19215
Helfenbein KG, Fourcade HM, Vanjani RG, Boore JL (2004) The mitochondrial genome of Paraspadella gotoi is highly reduced and reveals that chaetognaths are a sister group to protostomes. Proc Natl Acad Sci USA 101:10639–10643
Helm MH, Brule F, Degoul F, Cepanec C, Leroux J-P, Geige R, Florentz C (1998) The presence of modified nucleotides is required for cloverleaf folding in a human mitochondrial tRNA. Nucl Acids Res 26:1636–1643
Juhling F, Morl M, Hartmann RK, Sprinzl M, Stadler PF, Putz J (2009) Compilation of tRNA sequences and tRNA genes. Nucleic Acids Res 37(1):D159–D162
Kayal E, Lavrov DV (2008) The mitochondrial genome of Hydra oligactis (Cnidaria, Hydrozoa) sheds new light on animal mtDNA evolution and cnidarian phylogeny. Gene 410:177–186
Lavrov DV (2007) Key transitions in animal evolution: a mitochondrial DNA perspective. Integr Comp Biol 47:734–774
Lavrov DV (2011) Key transitions in animal evolution: a mitochondrial DNA perspective. In: Desalle R, Schierwater B (eds) Key transitions in animal evolution. Science publishers & CRS Press, Enfield, pp 35–54
Lill R, Lepier A, Schwagele F, Sprinzl M, Vogt H, Wintermeyer W (1988) Specific recognition of the 3′-terminal adenosine of tRNAPhe in the exit site of E. coli ribosomes. J Mol Biol 203:699–705
Limbach PA, Crain PF, McCloskey JA (1994) Summary: the modified nucleosides of RNA. Nucl Acids Res 22:2183–2196
Liu M, Horowitz J (1994) Functional transfer RNAs with modifications in the 3′-CCA end: differential effects on aminoacylation and polypeptide synthesis. Proc Natl Acad Sci USA 91:10389–10393
Marechal-Drouard L, Guillemaut P, Cosset A, Arbogast M, Weber F, Weil JH, Dietrich A (1990) Transfer RNAs of potato (Solanum tuberosum) mitochondria have different genetic origins. Nucl Acids Res 18:3689–3696
Marechal-Drouard L, Weil JH, Guillemaut P (1988) Import of several tRNAs from the cytoplasm into the mitochondria in bean Phaseolus vulgaris. Nucl Acids Res 16:4777–4788
Marck C, Grosjean H (2002) tRNomics: analysis of tRNA genes from 50 genomes of Eukarya, Archaea, and Bacteria reveals anticodon-sparing strategies and domain-specific features. RNA 8:1189–1232
Martin NC (1995) Organellar tRNAs: biosynthesis and function. In: Soll D, RajBhandary UL (eds) tRNA: structure, biosynthesis, and function. ASM press, Washington DC, pp 127–140
Medina M, Collins AG, Takaoka TL, Kuehl JV, Boore JL (2006) Naked corals: skeleton loss in Scleractinia. Proc Natl Acad Sci 103:9096–9100
Montiel R, Lucena MA, Medeiros J, Simoes N (2006) The complete mitochondrial genome of the entomopathogenic nematode Steinernema carpocapsae: insights into nematode mitochondrial DNA evolution and phylogeny. J Mol Evol 62:211–225
Nagaike T, Suzuki T, Tomari Y, Takemoto-Hori C, Negayama F, Watanabe K, Ueda T (2001) Identification and characterization of mammalian mitochondrial tRNA nucleotidyltransferases. J Biol Chem 276:40041–40049
Okimoto R, Macfarlane JL, Wolstenholme DR (1990) A set of tRNAs that lack either the TψC arm or the dihydrouridine arm: towards a minimal tRNA adaptor. EMBO J 9:3405–3411
Okimoto R, Macfarlane JL, Clary DO, Wolstenholme DR (1992) The mitochondrial genomes of two nematodes, Caenorhabditis elegans and Ascaris suum. Genetics 130:471–498
Pont-Kingdon GA, Beagley CT, Okimoto R, Wolstenholme DR (1994) Mitochondrial DNA of the sea anemone, Metridium senile (Cnidaria): prokaryote-like gene for tRNAf-Met and small subunit ribosomal RNA, and standard genetic code specificities for AGR and ATA codons. J Mol Evol 39:387–399
Pont-Kingdon GA, Vassort CG, Warrior R, Okimoto R, Beagley CT, Wolstenholme DR (2000) Mitochondrial DNA of Hydra attenuata (Cnidaria): a sequence that includes an end of one linear molecule and the genes for l-rRNA, tRNAf-Met, tRNATrp, COII, and ATPase8. J Mol Evol 51:404–415
Pont-Kingdon GA, Okada NA, Macfarlane JL, Beagley CT, Watkins-Sims CD, Cavalier-Smith T, Clark-Walker GD, Wolstenholme DR (1998) Mitochondrial DNA of the coral Sarcophyton glaucum contains a gene for a homologue of bacterial MutS: a possible case of gene transfer from the nucleus to the mitochondrion. J Mol Evol 46:419–443
RajBhandary UL, Chow CM (1995) Initiator tRNAs and the initiation of protein synthesis. In: Soll D, RajBhandary UL (eds) tRNA: structure, biosynthesis, and function. ASM press, Washington DC, pp 511–528
Randerath E, Hari P, Agrawal HP, Randerath K (1981) Rat liver mitochondrial lysine tRNA (anticodon U*UU) contains a rudimentary D-arm and 2 hypermodified nucleotides in its anticodon loop. Biochem Biophys Res Commun 103:739–744
Rickwood D, Wilson MT, Darley-Usmar VM (1987) Isolation and characteristics of intact mitochondria. In: Darley-Usmar VM, Rickwood D, Wilson MT (eds) Mitochondria: a practical approach. IRL Press, Washington DC, pp 1–16
Roe BA, Wong JFH, Chen EY, Armstrong PW, Stankeiwicz A, Ma D-P, McDonough J (1982) Mammalian mitochondrial tRNAs: a modified nucleotide 3′ to the anticodon may modulate their codon response. In: Slonimsky P, Borst P, Attardi G (eds) Mitochondrial genes. Cold Spring Harbor Laboratory, Cold Spring Harbor, pp 45–49
Samaha RR, Green R, Noller HF (1995) A base pair between tRNA and 23S rRNA in the peptidyl transferase centre of the ribosome. Nature (London) 377:309–314
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schneider A (2011) Mitochondrial tRNA import and its consequences for mitochondrial translation. Ann Rev Biochem 80:1033–1053
Simpson AM, Suyama Y, Dewes H, Campbell DA, Simpson L (1989) Kinetoplastid mitochondria contain functional tRNAs which are encoded in nuclear DNA and also contain small minicircle and maxicircle transcripts of unknown function. Nucl Acids Res 17:5427–5445
Shao Z, Graf S, Chaga OY, Lavrov DV (2006) Mitochondrial genome of the moon jelly Aurelia aurita (Cnidaria, Scyphozoa): a linear DNA molecule encoding a putative DNA-dependant DNA polymerase. Gene 381:92–101
Sprinzl M, Horn C, Brown M, Ioudovitch A, Teinberg S (2005) Compilation of tRNA sequences and sequences of tRNA genes. Nucl Acids Res 26:148–153
Tamura K (1994) The role of the CCA sequence of tRNA in the peptidyl-transfer reaction. FEBS Lett 353:173–176
Tamura K, Nameki N, Hasegawa T, Shimizu M, Himeno H (1994) Role of the CCA terminal sequence of tRNAVal in aminoacylation with valyl-tRNA synthetase. J Biol Chem 269:22173–22177
Tarassov I, Komenski P, Kolesnikova O, Martin RP, Krasheninnikov IA, Entelis N (2007) Import of nuclear DNA-encoded RNAs into mitochondria and mitochondrial translation. Cell Cycle 6(2473):2477
Tomita K, Weiner AM (2001) Collaboration between CC- and A-adding enzymes to build and repair the 3′-terminal CCA of tRNA in Aquifex aeolicus. Science 294:1334–1336
van Oppen MJ, Catmull J, McDonald BJ, Hislop NR, Hagerman PJ, Miller DJ (2002) The mitochondrial genome of Acropora tenuis (Cnidaria; Scleractinia) contains a large group I intron and a candidate control region. J Mol Evol 55:1–13
Watanabe K, Yokobori S (2011) tRNA modification and genetic code variations in animal mitochondria. J Nucl Acids 623095
Wolstenholme DR (1992) Animal mitochondrial DNA: structure and evolution. In: Wolstenholme DR, Jeon KW (eds) Mitochondria genomes. International review of cytology, vol 141. Academic Press, New York, pp 173–216
Wolstenholme DR, Macfarlane JL, Okimoto R, Clary DO, Wahleithner JA (1987) Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci USA 84:1324–1328
Wolstenholme DR, Okimoto R, Macfarlane JL (1994) Nucleotide correlations that suggest tertiary interactions in the TV-replacement loop-containing mitochondrial tRNAs of the nematodes, Caenorhabditis elegans and Ascaris suum. Nucl Acids Res 22:4300–4306
Wolstenholme DR, Fauron CM-R (1995) Mitochondrial genome organization. In Levings III CS, Kluwer VIK (eds) Advances in cellular and molecular biology of plants. Molecular biology of the mitochondria, vol 3. Academic Publishers, the Netherlands, pp 1–59
Acknowledgments
We thank Michael Bastiani, Brenda L. Bass, Raymond F. Gesteland, David P. Goldenberg for discussions during the course of this work, and Robert Schackmann for oligonucleotides (partially subsidized by National Institutes of Health Grant CA-42014) This work was supported by National Institutes of Health Grant GM-18375 and funds from the University of Utah, and was submitted by C. T. Beagley in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Biology), College of Science, University of Utah.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Hohmann.
Rights and permissions
About this article
Cite this article
Beagley, C.T., Wolstenholme, D.R. Characterization and localization of mitochondrial DNA-encoded tRNAs and nuclear DNA-encoded tRNAs in the sea anemone Metridium senile . Curr Genet 59, 139–152 (2013). https://doi.org/10.1007/s00294-013-0395-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-013-0395-9