Abstract
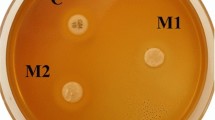
In the present study, a composite composed of resin containing imidazole-GMA resin and a natural filler of silk powder (IGMSP) was used as an alternative to bisphenol A-glycidyl methacrylate (Bis-GMA) dental composites. Dental composite was prepared by both optical and thermal polymerizations. FTIR, 1HNMR spectroscopy, and FESEM-EDX were used to identify the structure and morphology of the synthesized compounds. Thereafter, the thermal properties, water sorption and solubility, and anti-cancer and antibacterial activities of the composite were investigated. The structure of Imidazole-GMA resin as well as the successful surface modification of the filler with Si–O groups were confirmed with 1HNMR and FTIR spectra. Due to the use of natural filler of silk powder, the thermal resistance of the composite was found to be relatively low. The water adsorption and solubility of composite were lower than Bis-GMA. The results of antibacterial properties and cytotoxicity of the composite were much promising due to the presence of imidazole components in the resin structure. This composite was shown to have a significant inhibitory effect on MCF7 cells (IC50 = 11.4 µM, Maximum inhibitory effect 94.68%), and it was proved that Imidazole-GMA has significant effects on all three gram-negative (IZD 27–35 mm) and three gram-positive (IZD 22–29 mm) bacterial species.
Graphical abstract










Similar content being viewed by others
References
Ngren U (2001) Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. J Oral Rehabil 28:1106–1115
Xu Y, Wang H, Xie D (2018) Preparation of new low viscosity urethane dimethacrylates for dental composites. J Biomater Sci Polym Ed 29:1011–1025
He J, Luo Y, Liu F, Jia D (2010) Synthesis, characterization and photopolymerization of a new dimethacrylate monomer based on (α-methyl-benzylidene)bisphenol used as root canal sealer. J Biomater Sci Polym Ed 21:1191–1205
Stupp SI, Weldert SC (1984) Electrostatic phenomena and molecular motion at interfaces of glass/polymer composites. Polym Compos 5:224–230
Valadez-González A, Rosales-Ibáñez R, Rodríguez-Navarrete A, Villamar-Duque TE, Cano-Brown J, Carrillo-Escalante HJ, Ortiz-Fernández A, Hernández-Sánchez F (2021) Tailoring surface properties of carbon nanofibers via oxidation and its influence on dental pulp stem cell viability of PCL/CNF composites. Polym Bull 78:695–711
Silvério HA, Perin Leite AR, Dias da Silva MD, Nascimento de Assunção RM, Pero AC, Pasquini D (2021) Poly (ethyl methacrylate) composites reinforced with modified and unmodified cellulose nanocrystals and its application as a denture resin. Polym Bull. https://doi.org/10.1007/s00289-021-03621-0
Catalán A, Martínez A, Muñoz C, Medina C, Marzialetti T, Montaño M, Jaramillo AF, Meléndrez MF (2022) The effect of preheating of nano-filler composite resins on their degree of conversion and microfiltration in dental fillings. Polym Bull. https://doi.org/10.1007/s00289-021-03880-x
Jandt KD, Sigusch BW (2009) Future perspectives of resin-based dental materials. Dent Mater 25(8):1001–1006
Jandt KD, Mills RW (2013) A brief history of LED photopolymerization. Dent Mater 29:605–617
Krishnakumar S, Senthilvelan T (2019) Polymer composites in dentistry and orthopedic applications-a review. Mater Today Proc 46:9707–9713
Barot T, Rawtani D, Kulkarni P, Akkireddyc S, Hussain CM (2020) Physicochemical and biological assessment of flow- able resin composites incorporated with farnesol loaded halloysite nanotubes for dental applications. J Mech Behav Biomed Mater 104:103675–103675
Ferracane JL (2011) Resin composite - State of the art. Dent Mater 27(1):29–38
He J, Garoushi S, Säilynoja E, Vallittu PK, Lassila L (2019) The effect of adding a new monomer ‘Phene’ on the polymerization shrinkage reduction of a dental resin composite. Dent Mater 35(4):627–635
Ferracane JL, Hilton TJ (2015) Polymerization stress—is it clinically meaningful. Dent Mater 32(1):1–10
Dijken JWVV, Lindberg A (2015) A 15-year randomized controlled study of a reduced shrinkage stress resin composite. Dent Mater 31(9):1150–1158
Kalachandra S, Sankarapandian M, Shobha HK, Taylor DF, Mcgrath JE (1997) Influence of hydrogen bonding on properties of BIS-GMA analogues. J Mater Sci Mater Med 8(5):283–286
Zha C (2019) Nanoindentation study on mechanical properties and curing depth of dental resin nanocomposites. Polym Compos 40:1473–1480
Kermanshahi S, Santerre JP, Cvitkovitch DG, Finer Y (2010) Biodegradation of resin-dentin interfaces increases bacterial microleakage. J Dent Res 89(9):996–1001
Li M, Mondrinos MJ, Chen X, Gandhi MR, Ko FK, Lelkes PI (2006) Elastin blends for tissue engineering scaffolds. J Biomed Mater Res Part A 79(4):963–973
Hansel C, Leyhausen G, Mai UEH, Geurtsen W (1998) Effects of various resin composite (Co)monomers and extracts on two caries-associated micro-organisms in vitro. J Dent Res 77(1):60–67
Luo S, Liu F, Yu B, He J (2019) Preparation of low shrinkage stress Bis-GMA free dental resin composites with a synthesized urethane dimethacrylate monomer. J Biomater Sci Polym Ed 30:137–149
Zhang Z (2017) Fluorene-9-bisphenol is anti-oestrogenic and may cause adverse pregnancy out- comes in mice. Nat Commun 8:1–13
Manoj MK, Ramakrishnan R, Babjee S, Nasim R (2018) High-performance liquid chromatography analysis of salivary bisphenol A levels from light-cured and chemically cured orthodontic adhesives. Am J Orthod Dentofac Orthop 154(6):803–803
Berge TLL, Lygre GB, Jönsson BAG, Lindh CH, Björkman L (2017) Bisphenol A concentration in human saliva related to dental polymer-based fillings. Clin Oral Investig 21(8):2561–2568
Podgórski M (2010) Synthesis and characterization of novel dimethacrylates of different chain lengths as possible dental resins. Dent Mater 26(6):188–194
Weinmann W, Thalacker C, Guggenberger R (2005) Siloranes in dental composites. Dent Mater 21(1):68–74
He J, Liu F, Vallittu PK, Lassila LVJ (2013) Synthesis and characterization of new dimethacrylate monomer and its application in dental resin. J Biomater Sci Polym Ed 24:417–430
Barszczewska-Rybarek IM (2009) Structure-property relationships in dimethacrylate networks based on Bis-GMA. UDMA and TEGDMA Dent Mater 25(9):1082–1089
Barkoula NM, Alcock B, Cabrera NO, Peijs T (2008) Flame-retardancy properties of intumescent ammonium poly(phosphate) and mineral filler magnesium hydroxide in combination with graphene. Polym Polym Compos 16:101–113
Duarte MLB, Medina LAR, Reyes PT, Pérez SEG, González AMH (2017) Biobased isosorbide methacrylate monomer as an alternative to bisphenol A glycerolate dimethacrylate for dental restorative appli- cations. J Appl Polym Sci 134(11):44591–44599
Pandey S, Rajput BS, Chikkali SH (2021) Refining plant oils and sugars to platform chemicals, monomers, and polymers. Green Chem 23:4255–4295
Łukaszczyk J, Janicki B, Frick A (2012) Investigation on synthesis and properties of isosorbide based bis-GMA analogue. J Mater Sci Mater Med 23(5):1149–1155
Ebel K, Koehler H, Gamer AO, Jäckh R (2000) Imidazole and derivatives. Encycl Ind Chem 18:637–645
Yurttaş L, Ertaş M, Çiftçi GA, Temel HE, Demirayak S (2017) Novel benzothiazole based imidazole derivatives as new cytotoxic agents against glioma (C6) and liver (HepG2) cancer cell lines. Acta Pharm Sci 55:39–47
Youngs WJ (2008) Anticancer activity of Ag(I) N-heterocyclic carbene complexes derived from 4,5-dichloro-1H-imidazole. Met Based Drugs 2008:1–7
Rademaker-Lakhai JM, Den V, Bongard D, Pluim D, Beijnen JH, Schellens JHM (2004) A phase I and pharmacological study with imidazolium-trans-DMSO-imidazole-tetrachlororuthenate, a novel ruthenium anti- cancer agent. Clin Cancer Res 10:3717–3727
Gałczyńska K (2019) Selective cytotoxicity and antifungal properties of copper(II) and cobalt(II) complexes with imidazole-4-acetate anion or 1-allylimidazole. Sci Rep 9:9777–9790
Rameshbabu AP, Mohanty S, Bankoti K, Ghosh P, Dhara S (2015) Effect of alumina, silk and ceria short fibers in reinforcement of Bis-GMA/TEGDMA dental resin. Compos Part B Eng 70:238–246
Vepari C, Kaplan DL (2007) Silk as a biomaterial. Prog Polym Sci 32(8–9):991–1007
Ghaemy M, Bazzar M, Berenjestanaki FR (2012) Curing of DGEBA/ZnO nanocomposite with new fluorinated curing agents: Study of kinetics, water absorption, thermal and photophysical properties. High Perform Polym 24(7):632–645
Bowen RL (1965) Method of preparing a monomer having phenoxy and methacrylate groups linked by hydroxy glyceryl groups. US Patented 3, 179, 623
Amininasab SM, Rashidi A, Taghavi M, Shami Z (2016) Preparation and characterization of novel thermostable polyamides bearing different photoactive pendent architectures with an- tibacterial properties. Chinese J Polym Sci (English Ed) 34:766–776
Wang T, Matinlinna JP, He J, Ahmed KE, Burrow MF (2020) Biomechanical and biological evaluations of novel BPA-free fibre-reinforced composites for biomedical applications. Mater Sci Eng C 117:111309–111309
Abdolmaleki S, Ghadermazi M, Fattahi A, Sheshmani S (2016) Synthesis, characterization, spectral studies and cytotoxic effects of mixed-ligand mono and binuclear copper(II) complexes and their amide ligands. Inorganica Chim Acta 443:284–298
Aliabadi A (2021) Investigation of X-ray crystal structure and in vitro cytotoxicity of two Ga(III) complexes containing pyridine dicarboxylic acid derivatives and 2-aminobenzimidazole. J Mol Struct 1223:129005–129005
Qin JL, Shen WY, Chen ZF, Zhao LF, Qin QP, Yu YC, Liang H (2017) Oxoaporphine metal complexes (CoII, NiII, ZnII) with high antitumor activity by inducing mitochondria-mediated apoptosis and S-phase arrest in HepG2. Sci Rep 7:46056–46056
Klancnik A, Piskernik S, Jersek B, Mozina SS (2010) Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J Microbiol Meth 81:121–126
Ellof JN (1998) A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med 64:711–713
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6:71–79
Derikvand Z, Dorosti N, Hassanzadeh F, Shokrollahi A, Mohammadpour Z, Azadbakht A (2012) Three new supramolecular compounds of copper (II), cobalt (II) and zirconium (IV) with pyridine-2,6-dicarboxylate and 3,4-diaminopyridine: Solid and solution states studies. Polyhedron 43:140–140
Wang X, Liu F, Lai J, Fu Z, You X (2014) Comparative investigations on the effects of pendent tri- fluoromethyl group to the properties of the polyimides containing diphenyl-substituted cyclopentyl Cardo-structure. J Fluor Chem 164:27–37
Qi J (2020) Single-crystal structure and intracellular localization of Zn(II)-thiosemicarbazone complex targeting mitochondrial apoptosis pathways. Bioorganic Med Chem Lett 30:127340–127340
Qiu YR, Zhang RF, Zhang SL, Cheng S, Li QL, Ma CL (2017) Novel organotin (IV) complexes derived from 4-fluorophenyl-selenoacetic acid: synthesis, characterization and in vitro cytostatic activity evaluation. New J Chem 41(13):5639–5650
Zhou DF (2011) Anticancer activity, attenuation on the absorption of calcium in mitochondria, and catalase activity for manganese complexes of N-substituted Di(picolyl)amine. Inorg Chem 50:6929–6937
Cao W, Qi J, Qian K, Tian L, Cheng Z, Wang Y (2019) Structure activity relationships of 2 quinolinecarboxaldehyde thiosemicarbazone gallium (III) complexes with potent and selective anticancer activity. J Inorg Biochem 191:174–182
Acknowledgements
We wish to express our gratitude to the Research Affairs Division of University of Kurdistan (UOK), Sanandaj (Iran), for partial financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amininasab, S.M., Ghoseiri, E. & Abdolmaleki, S. Synthesis of new resin containing imidazole as a dental composite based on silk powder and evaluation of its anti-cancer and antibacterial activity. Polym. Bull. (2024). https://doi.org/10.1007/s00289-024-05296-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00289-024-05296-9