Abstract
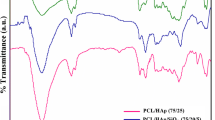
Bone is the second most common connective tissue worldwide and more than four million bone grafts or substitutes are used annually to treat bone defects. Due to the restrictions of using these therapies, today the development of bioactive 3D scaffolds has become a major field in bone tissue engineering. The main aim of this study was to fabricate polylactic acid/ polycaprolactone bone scaffolds by freeze extraction method. In order to improve the rheological, mechanical and biological properties of these scaffolds, hydroxyapatite, silica and their mixture were used as the filler loaded into scaffolds (35 wt%). All scaffolds were fabricated in three different concentrations of 5, 6.5 and 8% w/w based on polymer to investigate the effect of polymer concentration on their properties. In this regard, to evaluate the surface morphology of scaffolds and the size of pores, SEM analysis and porosity test were used. FTIR analysis was performed to identify functional groups and possible molecular interactions in the scaffold. The degree of degradation of scaffolds was conducted when incubated in PBS and also to better investigate the mechanical properties, a compression test was performed on cylindrical specimens. SEM images indicated that the pores were interconnected in the composite scaffolds and their size varied from 23 to 114 μm. The amount of porosity of the scaffolds made in this project ranged from 62.2 to 84.1%. These values demonstrated that the scaffolds prepared by the freeze extraction method had suitable pore sizes for use in tissue engineering. Considering the morphology and porosity as well as the appropriate pore size distribution in the 6.5% hybrid sample, this scaffold was selected as the optimal scaffold. The results indicated that the hydrophilicity of the scaffold surface was improved by the addition of silica nanoparticles and hydroxyapatite. The use of HA alone improved the mechanical properties of the porous structure, but the addition of SiO2 to HA more effectively strengthened the scaffolds. Furthermore, in samples containing silica, an increase in degradation was observed. According to the results of MTT test, the constructed scaffolds not only did not show cytotoxicity but also increased the rate of cell growth and proliferation. Pathological images showed that ECM deposition and collagen deposition were significantly higher in the hybrid groups from the third week onwards than in the other groups.



















Similar content being viewed by others
References
Turnbull G et al (2018) 3D bioactive composite scaffolds for bone tissue engineering. Bioactive materials 3(3):278–314
Sowjanya J et al (2013) Biocomposite scaffolds containing chitosan/alginate/nano-silica for bone tissue engineering. Colloids Surf, B 109:294–300
Guerzoni S et al (2014) Combination of silica nanoparticles with hydroxyapatite reinforces poly (L-lactide acid) scaffolds without loss of bioactivity. J Bioact Compat Polym 29(1):15–31
Yao Q et al (2017) Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 115:115–127
Shahrezaee M et al (2018) In vitro and in vivo investigation of PLA/PCL scaffold coated with metformin-loaded gelatin nanocarriers in regeneration of critical-sized bone defects. Nanomed: Nanotechnol, Biol Med 14(7):2061–2073
Roh H-S et al (2017) Addition of MgO nanoparticles and plasma surface treatment of three-dimensional printed polycaprolactone/hydroxyapatite scaffolds for improving bone regeneration. Mater Sci Eng, C 74:525–535
Puppi D et al (2010) Polymeric materials for bone and cartilage repair. Prog Polym Sci 35(4):403–440
Raeisdasteh Hokmabad V et al (2017) Design and fabrication of porous biodegradable scaffolds: a strategy for tissue engineering. J Biomater Sci Polym Ed 28(16):1797–1825
Hokmabad VR et al (2018) A comparison of the effects of silica and hydroxyapatite nanoparticles on poly (ε-caprolactone)-poly (ethylene glycol)-poly (ε-caprolactone)/chitosan Nanofibrous scaffolds for bone tissue engineering. Tissue Eng Regenerat Med 15(6):735–750
Hassanajili S et al (2019) Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater Sci Eng, C 104:109960
Tan W et al (2021) Dual-functional scaffolds of poly (L-lactic acid)/nanohydroxyapatite encapsulated with metformin: simultaneous enhancement of bone repair and bone tumor inhibition. Mater Sci Eng, C 120:111592
Ghorbani F, Zamanian A, Aidun A (2020) Conductive electrospun polyurethane-polyaniline scaffolds coated with poly (vinyl alcohol)-GPTMS under oxygen plasma surface modification. Mater Today Commun 22:100752
Stevens MM (2008) Biomaterials for bone tissue engineering. Mater Today 11(5):18–25
Budnicka M et al (2020) Poly-L-lactide scaffolds with super pores obtained by freeze-extraction method. J Biomed Mater Res B Appl Biomater 108(8):3162–3173
Sachlos E, Czernuszka J (2003) Making tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur Cell Mater 5(29):39–40
Chen G, Ushida T, Tateishi T (2002) Scaffold design for tissue engineering. Macromol Biosci 2(2):67–77
Cao Y et al (1997) Transplantation of Chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the. Plast Reconstr Surg 100:297–302
Freed LE et al (1993) Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J Biomed Mater Res 27(1):11–23
Madhumathi K et al (2009) Novel chitin/nanosilica composite scaffolds for bone tissue engineering applications. Int J Biol Macromol 45(3):289–292
Ghalia MA, Dahman Y (2017) Biodegradable poly (lactic acid)-based scaffolds: synthesis and biomedical applications. J Polym Res 24(5):1–22
Gentile P et al (2014) An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci 15(3):3640–3659
Martins C et al (2018) Poly (Lactic-co-Glycolic) acid: functionalizing PLGA and PLGA derivatives for drug delivery and tissue regeneration applications (Adv Healthcare Mater 1/2018). Adv Healthc Mater 7(1):1870002
Sartore L et al (2019) PLA/PCL-based foams as scaffolds for tissue engineering applications. Mater Today: Proceed 7:410–417
Hwang S, Todo M (2012) Characterization of compressive deformation behavior of multi-layer porous composite materials for articular tissue engineering. J Mech Sci Technol 26(7):1999–2004
Hajiali F, Tajbakhsh S, Shojaei A (2018) Fabrication and properties of polycaprolactone composites containing calcium phosphate-based ceramics and bioactive glasses in bone tissue engineering: a review. Polym Rev 58(1):164–207
Shu R et al (2003) Hydroxyapatite accelerates differentiation and suppresses growth of MC3T3-E1 osteoblasts. J Biomed Mater Res Part A: Off J Soc Biomateri, Japan Soc Biomater Austr Soc Biomater Korean Soc Biomater 67(4):1196–1204
Wutticharoenmongkol P, Pavasant P, Supaphol P (2007) Osteoblastic phenotype expression of MC3T3-E1 cultured on electrospun polycaprolactone fiber mats filled with hydroxyapatite nanoparticles. Biomacromol 8(8):2602–2610
Šupová M (2009) Problem of hydroxyapatite dispersion in polymer matrices: a review. J Mater Sci - Mater Med 20(6):1201–1213
Chen QZ, Thompson ID, Boccaccini AR (2006) 45S5 Bioglass®-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials 27(11):2414–2425
Porter JR, Ruckh TT, Popat KC (2009) Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol Prog 25(6):1539–1560
Chen G, Ushida T, Tateishi T (2000) A biodegradable hybrid sponge nested with collagen microsponges. J Biomed Mater Res: Off J Soc Biomater, Japan Soc Biomater Austr Soc Biomater Korean Soc Biomater 51(2):273–279
Ma J et al (2001) A preliminary in vitro study on the fabrication and tissue engineering applications of a novel chitosan bilayer material as a scaffold of human neofetal dermal fibroblasts. Biomaterials 22(4):331–336
Mooney DJ et al (1996) Novel approach to fabricate porous sponges of poly (D, L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials 17(14):1417–1422
Harris LD, Kim BS, Mooney DJ (1998) Open pore biodegradable matrices formed with gas foaming. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and the Australian Society for Biomaterials 42(3):396–402
Park YJ et al (1997) Porous poly (L-lactide) membranes for guided tissue regeneration and controlled drug delivery: membrane fabrication and characterization. J Control Release 43(2–3):151–160
Nam YS, Park TG (1999) Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J Biomed Mater Res: Off J Soci Biomater, Japanese Soc Biomater Austr Soci Biomater Korean Soc Biomater 47(1):8–17
Whang K et al (1995) A novel method to fabricate bioabsorbable scaffolds. Polymer 36(4):837–842
Han MJ (2007) Biodegradable membranes for the controlled release of progesterone. 1 Characterization of membrane morphologies coagulated from PLGA/progesterone/DMF solutions. J Appl Polymer Sci 75(1):60–67
Schugens C et al (1996) Biodegradable and macroporous polylactide implants for cell transplantation: 1. Preparation of macroporous polylactide supports by solid-liquid phase separation. Polymer 37(6):1027–1038
Ji C et al (2012) Fabrication of poly-DL-lactide/polyethylene glycol scaffolds using the gas foaming technique. Acta Biomater 8(2):570–578
Wu X et al (2010) Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomater 6(3):1167–1177
Sin D et al (2010) Polyurethane (PU) scaffolds prepared by solvent casting/particulate leaching (SCPL) combined with centrifugation. Mater Sci Eng, C 30(1):78–85
Montjovent M-O et al (2005) Biocompatibility of bioresorbable poly (L-lactic acid) composite scaffolds obtained by supercritical gas foaming with human fetal bone cells. Tissue Eng 11(11–12):1640–1649
Liu X et al (2009) Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials 30(12):2252–2258
Hao R et al (2011) Preparation and characterization of β-TCP/CS scaffolds by freeze-extraction and freeze-gelation. J Wuhan Univer Technol-Mater Sci Ed 26(2):371–375
Razak SIA et al (2016) A Conductive polylactic acid/polyaniline porous scaffold via freeze extraction for potential biomedical applications. Soft Mater 14(2):78–86
Ho M-H et al (2004) Preparation of porous scaffolds by using freeze-extraction and freeze-gelation methods. Biomaterials 25(1):129–138
Chen X et al (2019) 3D printed porous PLA/nHA composite scaffolds with enhanced osteogenesis and osteoconductivity in vivo for bone regeneration. Biomed Mater 14(6):065003
Rodenas-Rochina J, Ribelles JLG, Lebourg M (2013) Comparative study of PCL-HAp and PCL-bioglass composite scaffolds for bone tissue engineering. J Mater Sci - Mater Med 24(5):1293–1308
Kothapalli CR, Shaw MT, Wei M (2005) Biodegradable HA-PLA 3-D porous scaffolds: effect of nano-sized filler content on scaffold properties. Acta Biomater 1(6):653–662
Moghadam MZ et al (2017) Formation of porous HPCL/LPCL/HA scaffolds with supercritical CO2 gas foaming method. J Mech Behav Biomed Mater 69:115–127
Sun Y et al (2007) Culture of dermal fibroblasts and protein adsorption on block conetworks of poly (butyl methacrylate-block-(2, 3 propandiol-1-methacrylate-stat-ethandiol dimethacrylate)). Biomaterials 28(4):661–670
Jung S-C, Lee K, Kim B-H (2012) Biocompatibility of plasma polymerized sandblasted large grit and acid titanium surface. Thin Solid Films 521:150–154
Budyanto L, Goh Y, Ooi C (2009) Fabrication of porous poly (L-lactide)(PLLA) scaffolds for tissue engineering using liquid–liquid phase separation and freeze extraction. J Mater Sci - Mater Med 20(1):105–111
Hedayati F et al (2020) Preparation and properties of enhanced nanocomposites based on PLA/PC blends reinforced with silica nanoparticles. Polym Adv Technol 31(3):566–573
De Witte T-M et al (2018) Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regenerat Biomater 5(4):197–211
El-Kady AM et al (2010) Synthesis of silicate glass/poly (l-lactide) composite scaffolds by freeze-extraction technique: characterization and in vitro bioactivity evaluation. Ceram Int 36(3):995–1009
Diba M, Fathi M, Kharaziha M (2011) Novel forsterite/polycaprolactone nanocomposite scaffold for tissue engineering applications. Mater Lett 65(12):1931–1934
Diba M et al (2012) Preparation and characterization of polycaprolactone/forsterite nanocomposite porous scaffolds designed for bone tissue regeneration. Compos Sci Technol 72(6):716–723
Sinha R et al (2021) Additive manufactured scaffolds for bone tissue engineering: physical characterization of thermoplastic composites with functional fillers. ACS Appl Polymer Mater 3(8):3788–3799
Hollister SJ (2005) Porous scaffold design for tissue engineering. Nat Mater 4(7):518–524
Rizk HM, Al-Deen MSMS, Emam AA (2020) Comparative evaluation of Platelet Rich Plasma (PRP) versus Platelet Rich Fibrin (PRF) scaffolds in regenerative endodontic treatment of immature necrotic permanent maxillary central incisors: a double blinded randomized controlled trial. Saudi Dental J 32(5):224–231
Yee YY et al (2016) Preparation and characterization of poly (lactic acid)-based composite reinforced with oil palm empty fruit bunch fiber and nanosilica. BioResources 11(1):2269–2286
Park SH et al (2012) Scaffolds for bone tissue engineering fabricated from two different materials by the rapid prototyping technique: PCL versus PLGA. J Mater Sci - Mater Med 23(11):2671–2678
Dwivedi R et al (2020) Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J Oral Biol Craniof Res 10(1):381–388
Trindade R et al (2016) Foreign body reaction to biomaterials: on mechanisms for buildup and breakdown of osseointegration. Clin Implant Dent Relat Res 18(1):192–203
Onuki Y et al (2008) A review of the biocompatibility of implantable devices: current challenges to overcome foreign body response. J Diabetes Sci Technol 2(6):1003–1015
Anderson JM, Rodriguez A, Chang DT (2008) Foreign body reaction to biomaterials. Seminars in immunology. Elsevier, Amsterdam
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meyhami, T., Hassanajili, S., Tanideh, N. et al. Three dimensional scaffolds of hybrid PLA/PCL/HA/silica nanocomposites for bone tissue engineering. Polym. Bull. 81, 6025–6053 (2024). https://doi.org/10.1007/s00289-023-04978-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04978-0