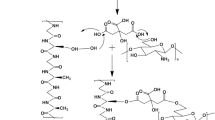
Abstract
For the synthesis of zinc oxide nanoparticles, a bioactive, cost-effective, and sustainable material such as the chitosan/silk fibroin blend was developed. The current research was aimed to elucidate the antibacterial activity of silk fibroin–chitosan blend ZnO NPs at various pH (4, 8 and 12) and temperatures (4, 37, and 50 °C) against gram-negative (Klebsiella pneumoniae, Escherichia coli, Serratia rubidaea, and Proteus mirabilis) and gram-positive bacterial strains (Staphylococcus aureus, Bacillus thuringensis, Bacillus licheniformis, and Clostridium difficile) at numerous concentrations (2–8 mg/ml). Agar well diffusion method was used to evaluate the antibacterial effect by measuring the zone of inhibition. Silk fibroin–chitosan blend ZnO NPs significantly reserved the growth of gram-negative bacterial strains, e.g., K. pneumoniae (11.3 ± 0.9 mm), E. coli (12.7 ± 0.9 mm), S. rubidaea (16.7 ± 0.9 mm), and P. mirabilis (11.7 ± 1.2 mm) and gram-positive bacterial strains such as B. thuringiensis (12.3 ± 0.3 mm), B. licheniformis (10.0 ± 0.57 mm), S. aureus (17.3 ± 1.2 mm), and C. difficile (11.7 ± 1.2 mm). At various temperatures and various pH values (acidic and basic), the stability of silk fibroin–chitosan blend ZnO NPs was observed. Silk fibroin–chitosan blend ZnO NPs significantly inhibited the growth of all bacterial strains at 37°C, and a maximum zone of inhibition was found at pH 8. The formation of SFCH blend ZnONPs was confirmed via numerous characterization techniques such as ultraviolet visible spectrum, Fourier transform infrared spectroscopy (FTIR), X-ray diffraction, and transmission electron microscopy (TEM). Transmission electron microscopy revealed that SFCH blend ZnONPs were highly stabilized and homogeneously distributed in the solution, and the average particle size was about 30.06 nm with a spherical shape. We concluded that silk fibroin–chitosan zinc oxide nanocomposite could be a safe and promising antimicrobial agent for wound dressing and various applications in biomaterials.
Graphical abstract











Similar content being viewed by others
References
Khyade VB, Yamanaka SJIJSRC (2018) Sericin from the cocoons of Silkworm, Antheraea mylitta (L) and Bombyx mori (L) for the reduction in hydrogen peroxide induced oxidative stress in feline fibroblasts. Int J Sci Res Chem 3(4):1–16.
Tsuchiya K, Masunaga H, Numata KJB (2017) Tensile reinforcement of silk films by the addition of telechelic-type polyalanine. Biomacromol 18(3):1002–1009
Yang Y et al (2015) Bioinspired porous octacalcium phosphate/silk fibroin composite coating materials prepared by electrochemical deposition. ACS Appl Mater Interfaces 7(10):5634–5642.
Wang X et al (2010) Silk nanospheres and microspheres from silk/pva blend films for drug delivery. Biomaterials 31(6):1025–1035
Nakazawa Y, Development of small-diameter vascular grafts based on silk fibroin fibers from Bombyx mori for vascular regeneration. et al (2011) J Biomater Sci Polymer Edn 22(1–3):195–206
Basal G et al (2010) Antibacterial properties of silk fibroin/chitosan blend films loaded with plant extract. Fibers Polymers 11(1):21–27.
Sashina E et al (2007) Compatibility of fibroin/chitosan and fibroin/cellulose blends studied by thermal analysis. J Thermal Anal Calorimetry 89(3):887–891.
Wongpinyochit T et al (2015) PEGylated silk nanoparticles for anticancer drug delivery. Biomacromol 16(11):3712–3722
Zhong T et al (2015) Silk fibroin/copolymer composite hydrogels for the controlled and sustained release of hydrophobic/hydrophilic drugs. Int J Pharmaceut 494(1):264–270.
Mhuka V et al (2013) Fabrication and structural characterization of electrospun nanofibres from Gonometa postica and Gonometa rufobrunnae regenerated silk fibroin. Macromolecular Res 21(9):995–1003.
Defrates K et al (2018) Protein polymer-based nanoparticles: fabrication and medical applications. Int J Molec Sci 19(6):1717.
Mosallanezhad P et al (2022) Fabrication and characterization of polycaprolactone/chitosan nanofibers containing antibacterial agents of curcumin and ZnO nanoparticles for use as wound dressing. Front Bioeng Biotechnol 10 (5):1027351.
Belbekhouche S et al (2019) Chitosan based self-assembled nanocapsules as antibacterial agent. Colloids Surf B 181:158–165
Arif M et al (2021) A complete characterization of microalgal biomass through FTIR/TGA/CHNS analysis: An approach for biofuel generation and nutrients removal. Renew Energy 163:1973–1982
Xu T et al (2010) Synthesis, characteristic and antibacterial activity of N,N,N-trimethyl chitosan and its carboxymethyl derivatives. Carbohyd Polym 81(4):931–936
Khan F et al (2020) Chitosan and their derivatives: antibiofilm drugs against pathogenic bacteria. Colloids Surfaces B Biointerfaces 185:110627.
Kabiri M et al (2021) The dry powder formulation of mixed cross-linked dextran microspheres and tetanus toxoid-loaded trimethyl chitosan nanospheres as a potent adjuvant for nasal delivery system. Iran J Basic Med Sci 24(1):116–122.
Jonassen H et al (2012) Effects of ionic strength on the size and compactness of chitosan nanoparticles. Colloid Polymer Sci 290(10):919–929.
Korniienko V et al. (2022) Impact of electrospinning parameters and post-treatment method on the antibacterial and antibiofilm activity of chitosan nanofibers. Molecules 27(10):3343.
Salahuddin N, El‐Kemary M, Ibrahim EJIJOACT (2018) Reinforcement of polymethyl methacrylate denture base resin with ZnO nanostructures. Int J Appl Ceram Technol 15(2):448–459.
Duffy LL et al (2018) Investigation into the antibacterial activity of silver, zinc oxide and copper oxide nanoparticles against poultry-relevant isolates of Salmonella and Campylobacter. Food Control 92:293–300
Siddiqi KS, Rahman A, Husen A (2018) Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res Lett 13(1):1–13.
Fu F et al (2015) Construction of cellulose based ZnO nanocomposite films with antibacterial properties through one-step coagulation. ACS Appl Mater Interfaces 7(4): 2597–2606.
Sirelkhatim A et al (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-micro Lett 7(3):219–242.
Sivakumar P et al (2018) Photo-triggered antibacterial and anticancer activities of zinc oxide nanoparticles. J Mater Chem B 6(30):4852–4871.
Malik GK, Mitra JJFB (2021) Zinc oxide nanoparticle synthesis, characterization, and their effect on mechanical, barrier, and optical properties of HPMC-based edible film. Food Bioprocess Technol 14(3):441–456.
Singh P et al (2018) Antimicrobial effects of biogenic nanoparticles. Nanomaterials 8(12):1009.
Labhane PK et al (2015) Synthesis of Cu doped ZnO nanoparticles: crystallographic, optical, FTIR, morphological and photocatalytic study. J Mater Sci Chem Eng 3(07):39.
Hosamani G, Kariyajjanavar P (2019) Optical and electrical properties of Cu–ZnO prism shaped nanocrystals by microwave combustion method. Am Energy Res 7(1): 31–40.
Zuluaga-Vélez A et al (2019) Silk fibroin hydrogels from the Colombian silkworm Bombyx mori L: evaluation of physicochemical properties. PLoS One 14(3):e0213303.
Bhatt VK et al (2020) Enhanced antifungal activity of WS2/ZnO nanohybrid against Candida albicans. ACS Biomateri Sci Eng 6(11):6069–6075.
Kumar S et al (2020) Biodegradable hybrid nanocomposite of chitosan/gelatin and green synthesized zinc oxide nanoparticles for food packaging. Foods 9(9):1143.
Nicoara AI et al (2020) In situ and ex situ designed hydroxyapatite: bacterial cellulose materials with biomedical applications. Materials 13(21):4793.
Jin S-E, Jin H-E (2021) Antimicrobial activity of zinc oxide nano/microparticles and their combinations against pathogenic microorganisms for biomedical applications: from physicochemical characteristics to pharmacological aspects. Nanomaterials 11(2):263–274
Yusof NAA, Zain NM, Pauzi N (2019) Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against Gram-positive and Gram-negative bacteria. Int J Biol Macromol 124:1132–1136
Lai RWS et al (2020) Temperature and salinity jointly drive the toxicity of zinc oxide nanoparticles: a challenge to environmental risk assessment under global climate change. Environ Sci Nano 7(10):2995–3006
Mutlaq S et al (2021) Conductometric immunosensor for Escherichia coli O157: H7 detection based on polyaniline/zinc oxide (PANI/ZnO) nanocomposite. Polymers 13(19):3288.
Mumtaz S et al (2021) Evaluation of antibacterial activity of vitamin c against human bacterial pathogens. Braz J Biol 83.
Muhammad Tahir H et al (2020) Synthesis of sericin‐conjugated silver nanoparticles and their potential antimicrobial activity. J Basic Microbiol 60(5):458–467.
Pandey V et al (2020) Silk as a leading-edge biological macromolecule for improved drug delivery. J Drug Deliv Sci Technol 55:101294.
Wang Z et al (2019) Effect of silk degumming on the structure and properties of silk fibroin. J Textile Institute 110(1):134–140.
Tariq M et al (2021) Silk derived formulations for accelerated wound healing in diabetic mice. Peer J 9:e102–e132
Siavashani AZ et al (2019) Silk based scaffolds with immunomodulatory capacity: anti-inflammatory effects of nicotinic acid. J Mater Sci 8(1):148–162.
Shahbazi B et al (2015) Preparation and characterization of silk fibroin/oligochitosan nanoparticles for siRNA delivery. Colloids Surf B 136:867–877
Gokila S et al (2018) Development of 3D scaffolds using nanochitosan/silk-fibroin/hyaluronic acid biomaterials for tissue engineering applications. Int J Biol Macromol 120:876–885
Salama AH (2018) Chitosan/silk fibroin/zinc oxide nanocomposite as a sustainable and antimicrobial biomaterial. Cell Chem Technol 52(9–10):903–907
Perera W et al (2020) Curcumin loaded zinc oxide nanoparticles for activity-enhanced antibacterial and anticancer applications. RSC Adv 10(51):30785–30795.
Bharathi D et al (2019) Preparation of chitosan coated zinc oxide nanocomposite for enhanced antibacterial and photocatalytic activity: As a bionanocomposite. Int J Biol Macromol 129:989–996
Mehrabani MG et al (2018) Preparation of biocompatible and biodegradable silk fibroin/chitin/silver nanoparticles 3D scaffolds as a bandage for antimicrobial wound dressing. Int J Biol Macromolecules 114:961–971.
Salama A (2018) Chitosan/silk fibroin/zinc oxide nanocomposite as a sustainable and antimicrobial biomaterial. Cell Chem Technol 52:903–907.
Marín CB et al (2020) Silk polymers and nanoparticles: a powerful combination for the design of versatile biomaterials. Front Chem 8:1141.
Farzana R et al (2017) Antimicrobial behavior of zinc oxide nanoparticles and β-lactam antibiotics against pathogenic bacteria. Arch Clin Microbiolol 8:57.
Bakil SNA et al (2020) Sodium alginate-zinc oxide nanocomposite film for antibacterial wound healing applications. Biointerface Res Appl Chem 10:6289–6296.
Mahamuni PP et al (2019) Synthesis and characterization of zinc oxide nanoparticles by using polyol chemistry for their antimicrobial and antibiofilm activity. Biochem Biophys Reports 17:71–80
Jafarzadeh S et al (2020) Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci Technol 1(100):262–277.
Majumder S et al (2020) Zinc oxide nanoparticles functionalized on hydrogel grafted silk fibroin fabrics as efficient composite dressing. Biomolecules 10(5):710.
Wang YC et al (2018) Evaluation of a series of silk fibroin protein‐based nonwoven mats for use as an anti‐adhesion patch for wound management in robotic surgery. J Biomed Mater Res A 106(1):221–230.
Revathi T et al (2018) Immobilization of ZnO on Chitosan-Neem seed composite for enhanced thermal and antibacterial activity. Adv Powder Technol 29(6):1445–1454.
Pesika NS et al (2003) Determination of the particle size distribution of quantum nanocrystals from absorbance spectra. Adv Mater 15(15):1289–1291.
Tajdari A, Babaei A et al (2021) Hybridization as an efficient strategy for enhancing the performance of polymer nanocomposites. Polymer Compos 42(12):6801–6815.
Babaei A, Haji M et al (2022) Polylactic acid/polycaprolactone bionanocomposites containing zinc oxide nanoparticles: Structure, characterization and cytotoxicity assay. J Thermoplastic Compos Mater 14(6):08927057221118823.
Mulisa E, Asres K, Engidawork E (2015) Evaluation of wound healing and anti-inflammatory activity of the rhizomes of Rumex abyssinicus J.(Polygonaceae) in mice. BMC Complement Altern Med 15(1):1–10.
Alyamani AA, Albukhaty S, Aloufi S, AlMalki FA, Al-Karagoly H, Sulaiman GM (2021) Green fabrication of zinc oxide nanoparticles using phlomis leaf extract: characterization and in vitro evaluation of cytotoxicity and antibacterial properties. Molecules 26(20):6140
Xie Y, He Y, Irwin PL, Jin T, Shi X (2011) Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl Environ Microbiol 77(7):2325–2331
Leite-Silva VR, Le Lamer M, Sanchez WY, Liu DC, Sanchez WH, Morrow I, et al. (2013). The effect of formulation on the penetration of coated and uncoated zinc oxide nanoparticles into the viable epidermis of human skin in vivo. Euro J Pharmaceut Biopharmaceut 84(2):297–308.
Mohd Yusof H, Mohamad R, Zaidan UH, Abdul Rahman NA (2019) Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. J Anim Sci Biotechnol 10:1–22
Ghosh M, Sinha S, Jothiramajayam M, Jana A, Nag A, Mukherjee A (2016) Cyto-genotoxicity and oxidative stress induced by zinc oxide nanoparticle in human lymphocyte cells in vitro and Swiss albino male mice in vivo. Food Chem Toxicol 97:286–296
Acknowledgements
We would like to thank to Forest Department, Sericulture Wing, Punjab, Pakistan, for providing the silk cocoons in this study.
Author information
Authors and Affiliations
Contributions
SM and SA designed the experiment. Shumaila Mumtaz performed the experimentation with the guidance of SA. SM, HMT, and TAM reviewed the manuscript. SARL provided me with resources as well as a formal analysis of data. AH, MS, AZ, and SK reviewed and analyzed the data. Finally, all authors have read and accepted the document.
Corresponding author
Ethics declarations
Conflict of interest
The authors specified that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mumtaz, S., Ali, S., Tahir, H.M. et al. Biological applications of biogenic silk fibroin–chitosan blend zinc oxide nanoparticles. Polym. Bull. 81, 2933–2956 (2024). https://doi.org/10.1007/s00289-023-04865-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04865-8