Abstract
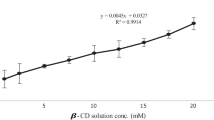
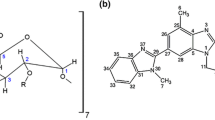
5-Fluorouracil is a chemotherapeutic agent used for the treatment of different kinds of cancers and Amlodipine Besylate is a vasodilator used to cure hypertension and angina. In order to improve different properties of drugs like drug solubility, mask bitter taste, prevent photodegradation, and for controlled drug delivery, the host–guest, solid inclusion complexes of 5-flurouracil (5-FU) and Amlodipine Besylate (ABS) with beta-cyclodextrin (β-CD) were utilized for different studies in this manuscript which were prepared through traditional methods and microwave-prompted technique. Different parameters such as apparent molar volume (Vϕ), partial molar volume at infinite dilution (Vϕ0), apparent molar isentropic compression (Kϕ,s), partial molar isentropic compression at infinite dilution (K0ϕ,s) were obtained from experimental density and speed of sound data. The partial molar volume at infinite dilution (Vϕ0 × 106/m3 mol−1) values of β-CD in aqueous solution of drug ABS was found 79.83, 80.32 and 80.64 at 305.15, 310.15 and 315.15 K, respectively. The partial molar volume at infinite dilution (Vϕ0 × 106/m3 mol−1) values of β-CD in aqueous solution of drug 5-FU was found − 252.61, − 246.42 and − 255.74 at 305.15, 310.15 and 315.15 K, respectively. The value of solubility constant (Ks) was found to be 74.3822 ± 4 M−1 for 5-FU and 204.0811 ± 4.75 M−1 for ABS in phase solubility studies. The inclusion complexes (ICs) were incorporated into the hybrid polymer networks (HPNs) of chitosan and gelatin to explore drug release studies. A comparison of directly loaded drug into the HPNs was made with the cyclodextrin-mediated release. The ICs prepared by different methods were also compared for the drug release.
Graphical abstract







Similar content being viewed by others
Abbreviations
- IC:
-
Inclusion complex
- HPN:
-
Hybrid polymer network
- ABS:
-
Amlodipine Besylate
- β-CD:
-
Beta cyclodextrin
- MW:
-
Microwave
- 5-FU:
-
5-Flurouracil
- CH:
-
Chitosan
- GEL:
-
Gelatin
References
Radu C, Parteni O, Ochiuz L (2016) Applications of cyclodextrins in medical textiles—review. J Control Release 224:146–157. https://doi.org/10.1016/j.jconrel.2015.12.046
Kacso I, Borodi G, Bende A, Bratu I (2012) Complexation of Amlodipine Besylate with β-cyclodextrin
Prabu S, Sivakumar K, Nayaki SK, Rajamohan R (2016) Host–guest interaction of cytidine in β-cyclodextrin microcavity: characterization and docking study. J Mol Liq 219:967–974. https://doi.org/10.1016/j.molliq.2016.04.017
Malakzadeh S, Alizadeh N (2018) Spectroscopic study and antioxidant activity of the inclusion complexes of cyclodextrins and amlodipine besylate drug. J Incl Phenom Macrocycl Chem 90:89–98. https://doi.org/10.1007/s10847-017-0768-7
Patil JS, Kadam DV, Marapur SC, Kamalapur MV (2010) Inclusion complex system; a novel technique to improve the solubility and bioavailability of poorly soluble drugs : a review. Int J Res Pharm Sci Rev Res 2:29–34. https://doi.org/10.1136/bmj.333.7574.873-a
Jin L, Liu Q, Sun Z et al (2010) Preparation of 5-fluorouracil/β-cyclodextrin complex intercalated in layered double hydroxide and the controlled drug release properties. Ind Eng Chem Res 49:11176–11181. https://doi.org/10.1021/ie100990z
European Medicines Agency (2014) Background review for cyclodextrins used as excipients. In the context of the revision of the guideline on ‘Excipients in the label and package leaflet of medicinal products for human use’
Gidwani B, Vyas A (2015) A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. Biomed Res Int. https://doi.org/10.1155/2015/198268
Kaur K, Jindal R, Jindal D (2020) Controlled release of vitamin B1 and evaluation of biodegradation studies of chitosan and gelatin based hydrogels. Int J Biol Macromol 146:987–999. https://doi.org/10.1016/j.ijbiomac.2019.09.223
Kaur K, Jindal R, Jindal D (2019) Synthesis, characterization and studies on host-guest interactions of inclusion complexes of metformin hydrochloride with β-cyclodextrin. J Mol Liq 282:162–168
Karmoker J, Joydhar P, Sarkar SRM (2016) Comparative in vitro evaluation of various commercial brands of amlodipine besylate tablets marketed in Bangladesh. Asian J Pharm Heal Sci 6:1384–1389
Ficarra R, Ficarra P, Di BMR et al (2000) Study of the inclusion complex of atenolol with beta-cyclodextrins. J Pharm Biomed Anal 23:231–236
Di Donato C, Lavorgna M, Fattorusso R et al (2016) Alpha- and beta-cyclodextrin inclusion complexes with 5-fluorouracil: characterization and cytotoxic activity evaluation. Molecules. https://doi.org/10.3390/molecules21121644
Buczek A, Staś M, Hebenstreit C et al (2021) Interaction of 5-fluorouracil with β-cyclodextrin: a density functional theory study with dispersion correction. Int J Quantum Chem 121:33–35. https://doi.org/10.1002/qua.26487
Bradea O, Kacso I, Borodi G et al (2012) Complexation of amlodipine besylate with β-cyclodextrin. Acta Chim Slov 59:18–23
Kapor A, Nikolić V, Nikolić L et al (2010) Inclusion complexes of amlodipine besylate and cyclodextrins. Cent Eur J Chem 8:834–841. https://doi.org/10.2478/s11532-010-0061-8
Miao J, Guo Z, Wang Y et al (2017) Study on the inclusion complex between β-cyclodextrin derivatives and flurbiprofen by spectrofluorometric. AIP Conf Proc doi 10(1063/1):4992818
Challa R, Ahuja A, Ali J, Khar RK (2005) Cyclodextrins in drug delivery : an updated review. AAPS PharmSciTech 6:329–357
Dalmolin MC, da Silva CE, Lunelli CE et al (2019) Modified β-cyclodextrin/amlodipine inclusion complexes: preparation and application in aqueous systems. J Mol Liq 276:531–540. https://doi.org/10.1016/j.molliq.2018.11.116
Jindal R (2021) RSM-CCD optimized microwave assisted synthesis of chitosan and sodium alginate based nanocomposite containing inclusion complexes of β-cyclodextrin and amlodipine besylate for sustained drug delivery systems. J Drug Deliv Sci Technol 61:102325. https://doi.org/10.1016/j.jddst.2021.102325
Kaur K, Jindal R, Jindal D (2018) RSM-CCD optimized microwave-assisted synthesis of chitosan and gelatin-based pH sensitive, inclusion complexes incorporated hydrogels and their use as controlled drug delivery systems. J Drug Deliv Sci Technol 48:161–173. https://doi.org/10.1016/j.jddst.2018.09.003
Kaur K, Jindal R (2018) Exploring RSM-CCD-optimized chitosan-/gelatin-based hybrid polymer network containing CPM-β-CD inclusion complexes as controlled drug delivery systems. Polym Bull. https://doi.org/10.1007/s00289-018-2555-z
Hiral M, Akhilesh D, Prabhakara P, Kamath JV (2012) Enhancement of solubility by complexation with cyclodextrin and nanocrystallisation. Int Res J Pharm 3:100–105
Cabral HM, Hadgraft J (1990) Studies of cyclodextrin inclusion complexes. I. The salbutamol-cyclodextrin complex as studied by phase solubility and -DSC. Int Res J Pharm 63:259–266
Loftsson T (2005) Evaluation of cyclodextrin solubilization of drugs. Int Res J Pharm 302:18–28. https://doi.org/10.1016/j.ijpharm.2005.05.042
Bibby DC, Davies NM, Tucker IG (2000) Mechanisms by which cyclodextrins modify drug release from polymeric drug delivery systems. Int J Pharm 197:1–11. https://doi.org/10.1016/S0378-5173(00)00335-5
Pessine FBT, Calderini A, Alexandrino GL (2012) Review: cyclodextrin inclusion complexes probed by NMR techniques. InTech, Rijeka
Machín R, Isasi JR, Vélaz I (2012) β-Cyclodextrin hydrogels as potential drug delivery systems. Carbohydr Polym 87:2024–2030. https://doi.org/10.1016/j.carbpol.2011.10.024
Higuchi T (1963) Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 52:1145–1149. https://doi.org/10.1002/jps.2600521210
Ritger PL, Peppas NA (1987) A simple equation for description of solute release. II. Fickian and anomalous release from swellable devices. J Control Release 5:37–42. https://doi.org/10.1016/0168-3659(87)90035-6
Bayraktar O, Malay Ö, Özgarip Y, Batigün A (2005) Silk fibroin as a novel coating material for controlled release of theophylline. Eur J Pharm Biopharm 60:373–381. https://doi.org/10.1016/j.ejpb.2005.02.002
Peppas NA, Sahlin JJ (1989) A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int J Pharm 57:169–172. https://doi.org/10.1016/0378-5173(89)90306-2
Das S, Subuddhi U (2013) Cyclodextrin mediated controlled release of naproxen from pH-sensitive chitosan/poly(vinyl alcohol) hydrogels for colon targeted delivery. Ind Eng Chem Res 52:14192–14200. https://doi.org/10.1021/ie402121f
Kumar H, Behal I (2016) Densities and speeds of sound of antibiotic drug chloramphenicol with l-leucine and glycyl-l-leucine in aqueous medium at T = (288.15–318.15) K : a volumetric, ultrasonic, and UV absorption study. J Chem Eng Data. https://doi.org/10.1021/acs.jced.6b00168
Kumar H, Kumar V, Sharma M, Behal I (2018) Studies on the interactions behaviour of polyhydroxy solutes d(+)-glucose and d(−)-fructose in aqueous triammonium citrate solutions over temperature range T = (288.15–318.15) K. J Chem Thermodyn 119:1–12. https://doi.org/10.1016/j.jct.2017.12.003
Mehra R, Yadav P (2012) Acoustic and volumetric studies of uracil in aqueous urea solution at different temperatures. Phys Chem Liq 50:88–101
Kumar H, Katal A (2018) Volumetric, acoustic and spectroscopic studies (FT-IR) of trisodium (TSC) and tripotassium citrate (TPC) in aqueous solution of ionic liquid 1-butyl-3-methylimidazolium tetrafluoro borate [C4mim][BF4] at different temperatures. J Chem Thermodyn 116:85–96. https://doi.org/10.1016/j.jct.2017.08.025
Bilensoy E, Çirpanli Y, Şen M et al (2007) Thermosensitive mucoadhesive gel formulation loaded with 5-Fu: cyclodextrin complex for HPV-induced cervical cancer. J Incl Phenom Macrocycl Chem 57:363–370. https://doi.org/10.1007/s10847-006-9259-y
Acknowledgements
The author is also grateful to the Materials research centre, MNIT Jaipur, for testing samples and DST-FIST for providing economic assistance for purchase of equipments like FTIR and UV-Visible spectrophotometer used in the characterization of the synthesized materials.
Funding
Financial assistance for a part of this research work was provided by Ministry of Education (Reg No. 15520006). Therefore, the author pays her gratitude to the organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that they have no conflict of interest with anyone in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, K., Jindal, R. A comparative study of the interactions of 5-fluorouracil and Amlodipine Besylate in aqueous β-cyclodextrin solution and drug release studies. Polym. Bull. 81, 2719–2740 (2024). https://doi.org/10.1007/s00289-023-04859-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04859-6