Abstract
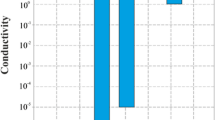
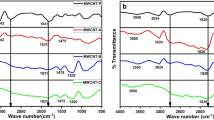
One of the promising directions in the field of creating new composite materials is the development of composites based on conductive polyaniline. In this work, synthesis of polyaniline was carried out in the presence of nanosized sulfur particles. Sulfur particles were obtained both in situ in the reaction mixture and added during polymerization. The studies of the process of oxidative polymerization of aniline in the presence of insoluble components were carried out using the method of monitoring the open circuit potential (OCP), as well as by recording the particle size with a laser analyzer. All obtained samples were studied using optical spectroscopy methods (IR-, UV-spectroscopy), elemental and thermogravimetric analyzes, scanning electron microscopy, and the change in conductivity depending on the percentage of sulfur was assessed. It was found that the presence of sulfur particles led to a significant decrease in the rate of aniline polymerization, which was reflected in the OCP profiles in the form of an increase in the time to reach maxima. Samples obtained using metal polysulfides demonstrate better thermal stability and electrical conduction properties.













Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Jadhav A, Qureshi SS, Jadhav H et al (2020) Conducting polymers and their composites. Contemp Nanomater Mater Eng Appl 147–178. https://doi.org/10.1007/978-3-030-62761-4
Molapo KM, Ndangili PM, Ajayi RF et al (2012) Electronics of conjugated polymers (I): polyaniline. Int J Electrochem Sci 7:11859–11875
Jangid NK, Jadoun S, Kaur N (2020) A review on high-throughput synthesis, deposition of thin films and properties of polyaniline. Eur Polym J 125:109485. https://doi.org/10.1016/j.eurpolymj.2020.109485
Sapurina I, Shishov M (2012) Oxidative polymerization of aniline: polyaniline molecular synthesis and the formation of supramolecular structures. In: Gomes AS (ed) New polymers for special applications. INTECH, pp 251−312. https://doi.org/10.5772/48758
Li L, Ruan G, Peng Z et al (2014) Enhanced cycling stability of lithium sulfur batteries using sulfur–polyaniline–graphene nanoribbon composite cathodes. ACS Appl Mater Interfaces 17:15033–15039. https://doi.org/10.1021/am5030116
Zhao X, Kim JK, Ahn HJ et al (2013) A ternary sulfur/polyaniline/carbon composite as cathode material for lithium sulfur batteries. Electrochim Acta 109:145–152. https://doi.org/10.1016/j.electacta.2013.07.067
Biglova Y, Salikhov R, Abdrakhmanov I et al (2017) Preparation and investigation of soluble functionalized polyanilines. Phys Solid State 59:1253–1259. https://doi.org/10.1134/S106378341706004X
Tamboli MS, Kulkarni MV, Patil RH et al (2012) Nanowires of silver–polyaniline nanocomposite synthesized via in situ polymerization and its novel functionality as an antibacterial agent. Colloids Surf B 92:35–41. https://doi.org/10.1016/j.colsurfb.2011.11.006
Tai H, Jiang Y, Xie G, Yu J (2010) Preparation, characterization and comparative NH3-sensing characteristic studies of PANI/inorganic oxides nanocomposite thin films. J Mater Sci Technol 26:605–613. https://doi.org/10.1016/S1005-0302(10)60093-X
Liu P, Liu W, Xue Q (2004) In situ chemical oxidative graft polymerization of aniline from silica nanoparticles. Mater Chem Phys 87:109–113. https://doi.org/10.1016/j.matchemphys.2004.05.001
He J, He Y, Fan Y et al (2017) Conjugated polymer-mediated synthesis of nitrogen-doped carbon nanoribbons for oxygen reduction reaction. Carbon 124:630–636. https://doi.org/10.1016/j.carbon.2017.08.081
Chang CH, Huang TC, Peng CW et al (2012) Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 50:5044–5051. https://doi.org/10.1016/j.carbon.2012.06.043
Wei P, Fan M, Chen H et al (2015) High-capacity graphene/sulfur/polyaniline ternary composite cathodes with stable cycling performance. Electrochim Acta 174:963–969. https://doi.org/10.1016/j.electacta.2015.06.052
Sapurina IY, Stejskal J, Trchová M et al (2006) Organic nanocolloidal polyaniline dispersions containing fullerene. Fuller Nanotub Carbon Nanostructures 14:447–455. https://doi.org/10.1080/15363830600666126
Zhou W, Yu Y, Chen H et al (2013) Yolk–shell structure of polyaniline-coated sulfur for lithium–sulfur batteries. J Am Chem Soc 135:16736–16743. https://doi.org/10.1021/ja409508q
Li W, Zhang Q, Zheng G et al (2013) Understanding the role of different conductive polymers in improving the nanostructured sulfur cathode performance. Nano Lett 13:5534–5540. https://doi.org/10.1021/nl403130h
Ghebache Z, Safidine Z, Hamidouche F et al (2021) Effect of hematite on the energy storage performance of polyaniline/zeolite HY/α-Fe2O3 nanocomposite supercapacitor electrode. J Inorg Organomet Polym Mater 31:1153–1162. https://doi.org/10.1007/s10904-020-01801-5
Benzerafa A, Amokrane S, Boulaouche T et al (2021) Synthesis of novel conducting polyaniline composites based on seaweed Enteromorpha compressa macro-alga powder. Polym Bull 78:1909–1924. https://doi.org/10.1007/s00289-020-03191-7
An Y, Wei P, Fan M et al (2016) Dual-shell hollow polyaniline/sulfur-core/polyaniline composites improving the capacity and cycle performance of lithium–sulfur batteries. Appl Surf Sci 375:215–222. https://doi.org/10.1016/j.apsusc.2016.03.070
Qiu L, Zhang S, Zhang L et al (2010) Preparation and enhanced electrochemical properties of nano-sulfur/poly(pyrrole-co-aniline) cathode material for lithium/sulfur batteries. Electrochim Acta 55:4632–4636. https://doi.org/10.1016/j.electacta.2010.03.030
Zhou W, Yu Y, Chen H et al (2013) Yolk−shell structure of polyaniline-coated sulfur for lithium−sulfur batteries. J Am Chem Soc 135:16736–16743. https://doi.org/10.1021/ja409508q
Mukhamedzyanova AA, Akhmetshin BS et al (2019) Obtaining sulfur nanoparticles by acid precipitation from an aqueous solution of calcium polysulfide. Reports of the Bashkir University 4:576–581. https://doi.org/10.33184/dokbsu-2019.6.1
Latypova LR et al (2020) Synthesis and physicochemical properties of poly [2-(2-chloro-1-methylbut-2-en-1-yl) aniline] obtained with various dopants. Polym Int 69:804–812. https://doi.org/10.1002/pi.6016
Mustafin AG, Sadykov TT et al (2020) A method for producing an electrically conductive composite based on polyaniline and nanoscale sulfur. RF patent application No. 2020143286, dated 25.12.2020. https://new.fips.ru/registers-doc-view/fips_servlet?DB=RUPATAP&DocNumber=2020143286
Massalimov IA, Mustafin AG, Shangareeva AR et al (2012) Method for producing colloidal nanosized sulfur. RF patent No. 2456231 dated 20.07.12. https://new.fips.ru/registers-doc-view/fips_servlet?DB=RUPAT&DocNumber=2456231
Andriianova AN, Biglova YN, Mustafin AG (2020) Effect of structural factors on the physicochemical properties of functionalized polyanilines. RSC Adv 10:7468–7491. https://doi.org/10.1039/C9RA08644G
Andriianova AN, Abyalilova AR, Biglova YuN et al (2020) Effect of cobalt phthalocyanine on the chemical polymerization of aniline. ChemistrySelect 5:5621–5628. https://doi.org/10.1002/slct.202001086
Ogurtsov NA, Mikhaylov SD, Coddeville P et al (2016) Influence of dispersed nanoparticles on the kinetics of formation and molecular mass of polyaniline. J Phys Chem B 120:10106–10113. https://doi.org/10.1021/acs.jpcb.6b05944
Ogurtsov NA, Noskov YV, Pud AA (2015) Effect of multiwalled carbon nanotubes on the kinetics of the aniline polymerization: the semi-quantitative OCP approach. J Phys Chem B 119:5055–5061. https://doi.org/10.1021/jp511665q
Voyutsky SS (1975) Course of Colloidal Chemistry, two ed., Chemistry, Moscow
Baibarac M, Baltog I, Lefrant S et al (2003) Polyaniline and carbon nanotubes based composites containing whole units and fragments of nanotubes. Chem Mater 15:4149–4156. https://doi.org/10.1021/cm021287x
Stejskal J, Sapurina I, Trchova M et al (1998) Solid-state protonation and electrical conductivity of polyaniline. Macromolecules 31:2218–2222. https://doi.org/10.1021/ma970823h
Huang WS, MacDiarmid AG (1993) Optical properties of polyaniline. Polymer 34:1833–1845. https://doi.org/10.1016/0032-3861(93)90424-9
Huang WS, Humphrey BD, MacDiarmid AG (1986) Polyaniline, a novel conducting polymer. Morphology and chemistry of its oxidation and reduction in aqueous electrolytes. J Chem Soc Faraday Trans 1 82:2385–2400. https://doi.org/10.1039/F19868202385
Masters JG, Sun Y, MacDiarmid AG et al (1991) Polyaniline: allowed oxidation states. Synth Met 41:715–718. https://doi.org/10.1016/0379-6779(91)91166-8
Sindhimeshram DC, Gupta MC (1995) Transport properties of substituted derivatives of polyaniline. Indian J Chem 34A:260–277
Tran HD, D’Arcy JM, Wang Y et al (2011) The oxidation of aniline to produce «polyaniline»: a process yielding many different nanoscale structures. J Mater Chem 21:3534–3550. https://doi.org/10.1039/C0JM02699A
Andriianova AN, Biglova YuN, Mustafin AG (2020) Metal phthalocyanines: effect on the synthesis and physicochemical properties of polyaniline. Mendeleev Commun 30:624–626. https://doi.org/10.1016/j.mencom.2020.09.024
Li X, Rao M, Li W (2016) Sulfur encapsulated in porous carbon nanospheres and coated with conductive polyaniline as cathode of lithium–sulfur battery. J Solid State Electrochem 20:153–161. https://doi.org/10.1007/s10008-015-3013-6
Wei Y, Focke WW, Wnek GE et al (1989) Synthesis and electrochemistry of alkyl ring-substituted polyanilines. J Phys Chem 93:495–499. https://doi.org/10.1021/j100338a095
Geniès EM, Lapkowski M, Penneau JF (1988) Cyclic voltammetry of polyaniline: interpretation of the middle peak. J Electroanal Chem Interfacial Electrochem 249:97–107. https://doi.org/10.1016/0022-0728(88)80351-6
Pruneanu S, Veress E, Marian I, Oniciu L (1999) Characterization of polyaniline by cyclic voltammetry and UV-Vis absorption spectroscopy. J Mater Sci 34:2733–2739. https://doi.org/10.1023/A:1004641908718
Li GC, Li GR, Ye SH, Gao XP (2012) A polyaniline-coated sulfur/carbon composite with an enhanced high-rate capability as a cathode material for lithium/sulfur batteries. Adv Energy Mater 2:1238–1245. https://doi.org/10.1002/aenm.201200017
Bhadra S, Khastgir D, Singha N, Lee J (2009) Progress in preparation, processing and applications of polyaniline. Prog Polym Sci 34:783–810. https://doi.org/10.1016/j.progpolymsci.2009.04.003
Acknowledgements
The study was carried out with the financial support of the Russian Foundation for Basic Research within the scientific project No. 20-33-90316\20. This work was supported by the Ministry of Science and Higher Education of the Russian Federation as part of the state task No. 1021062311390-1-1.4.1.
Author information
Authors and Affiliations
Contributions
Timur T. Sadykov: ideas, development or design of methodology, verification reproducibility of results, performing the experiments, writing—original draft preparation, preparation, creation of the published work; Ismail A. Massalimov: provision of study materials, reagents, materials, provision computing resources, or other analysis tools; Akhat G. Mustafin: ideas, provision of study materials, data curation, reagents, materials, supervision, project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sadykov, T.T., Massalimov, I.A. & Mustafin, A.G. Synthesis and physico-chemical properties of composites based on polyaniline and nanosized sulfur. Polym. Bull. 81, 2757–2776 (2024). https://doi.org/10.1007/s00289-023-04823-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04823-4