Abstract
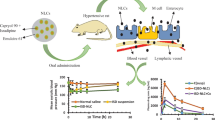
The incidence of pulmonary arterial hypertension (PAH) has significantly increased in the past few decades and therefore requires immediate attention. The applications of drug-loaded nano-systems have shown considerable improvements in controlling PAH compared to plain drugs. The purpose of this current investigation was to prepare and characterize sildenafil-encapsulated polyethylene glycol (PEGylated) liposomes for effective treatment of pulmonary arterial hypertension. Liposomes were prepared using thin film hydration method by varying the concentrations of sildenafil, Phospholipon 90-G, cholesterol, mPEG-DSPE2000 to optimize the formulation. The optimized liposomal formulation had mean hydrodynamic diameter, polydispersity index (PDI), zeta potential (ZP) and %EE of 155.3 ± 3.2 nm, 0.19 ± 0.1, − 42.2 ± 0.3 mV and 75.89 ± 0.5%. Transmission electron microscopy (TEM) revealed that liposomes were spherical and homogenously dispersed. FTIR (Fourier transform infrared spectroscopy) and DSC (Differential scanning calorimetry) confirmed that there was no covalent interaction between the drug and excipients. The release of sildenafil from PEGylated liposomes was found to be sustained compared to free drug. Ex vivo study in the rats showed improved vasorelaxant response for sildenafil loaded liposomes (132. 7 ± 0.7%) compared to pure sildenafil (108.2 ± 0.06%) and marketed Revatio® (113.2 ± 0.8%). In summary, sildenafil loaded PEGylated liposomes produced an impressive vasorelaxant response in rat aortic stip assay and require validation to bring the formulation available for clinical studies in the management of PAH.




Similar content being viewed by others
References
Galiè N et al (2015) Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 373(9):834–844
Licarete E, Sesarman A, Banciu M (2015) Exploitation of pleiotropic actions of statins by using tumour-targeted delivery systems. J Microencapsul 32:619–631
Thenappan T et al (2007) A USA-based registry for pulmonary arterial hypertension: 1982–2006. Eur Respir J 30(6):1103–1110
Chin KM et al (2018) psychometric validation of the pulmonary arterial hypertension-symptoms and impact (PAH-SYMPACT) questionnaire: results of the SYMPHONY trial. Chest 154(4):848–861
Lau EM et al (2017) Epidemiology and treatment of pulmonary arterial hypertension. Nat Rev Cardiol 14(10):603
Pramanik N et al (2019) Polyhydroxybutyrate-co-hydroxyvalerate copolymer modified graphite oxide based 3D scaffold for tissue engineering application. Mater Sci Eng C 94:534–546
Segura-Ibarra V et al (2018) Nanotherapeutics for treatment of pulmonary arterial hypertension. Front Physiol 9:890
Nahar K et al (2014) Peptide-coated liposomal fasudil enhances site specific vasodilation in pulmonary arterial hypertension. Mol Pharm 11(12):4374–4384
Chen L et al (2011) Nanoparticle-mediated delivery of pitavastatin into lungs ameliorates the development and induces regression of monocrotaline-induced pulmonary artery hypertension. Hypertension 57(2):343–350
Rafati N, Caldera F, Trotta F, Ghias N (2019) Pyromellitic dianhydride crosslinked cyclodextrin nanosponges for curcumin controlled release; formulation, physicochemical characterization and cytotoxicity investigations. J Microencapsul. https://doi.org/10.1080/02652048.2019.1669728
Shavi GV et al (2016) PEGylated liposomes of anastrozole for long-term treatment of breast cancer: in vitro and in vivo evaluation. J Liposome Res 26(1):28–46
Klibanov AL et al (1990) Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett 268(1):235–237
Musteata FM, Pawliszyn J (2006) Determination of free concentration of paclitaxel in liposome formulations. J Pharm Pharm Sci 9:231–237
Sudhakar B, Krishna MC, Murthy KVR (2016) Factorial design studies of antiretroviral drug-loaded stealth liposomal injectable: PEGylation, lyophilization and pharmacokinetic studies. Appl Nanosci 6(1):43–60
Yang X et al (2009) A novel liposomal formulation of flavopiridol. Int J Pharm 365(1–2):170–174
Dave V et al (2017) Hybrid nanoparticles for the topical delivery of norfloxacin for the effective treatment of bacterial infection produced after burn. J Microencapsul 34(4):351–365
Shah SP, Misra A (2004) Liposomal amikacin dry powder inhaler: effect of fines on in vitro performance. AAPS PharmSciTech 5(4):107–113
Lum W et al (2017) Novel liposome-based surface-enhanced raman spectroscopy (SERS) substrate. J Phys Chem Lett 8(12):2639–2646
Biltonen RL, Lichtenberg D (1993) The use of differential scanning calorimetry as a tool to characterize liposome preparations. Chem Phys Lipid 64(1–3):129–142
Dave V et al (2017) Herbal liposome for the topical delivery of ketoconazole for the effective treatment of seborrheic dermatitis. Appl Nanosci 7(8):973–987
Korsmeyer RW et al (1983) Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 15(1):25–35
Tripathi N et al (2018) Discovery of novel soluble epoxide hydrolase inhibitors as potent vasodilators. Sci Rep 8(1):1–11
Mojzych M et al (2016) Relaxant effects of selected sildenafil analogues in the rat aorta. J Enzyme Inhib Med Chem 31(3):381–388
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Paliwal, S., Sharma, J., Dave, V. et al. Novel biocompatible polymer-modified liposome nanoparticles for biomedical applications. Polym. Bull. 81, 535–547 (2024). https://doi.org/10.1007/s00289-023-04731-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04731-7