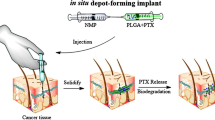
Abstract
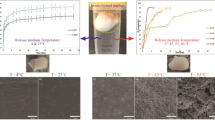
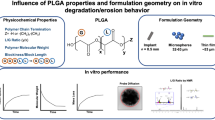
In situ forming implants (ISFI) based on poly(lactic-co-glycolic acid) (PLGA) are promising long-acting injectable depots for the treatment of human immunodeficiency virus-1 infection. One of the key parameters of depot formulations is the drug release rate which is critical for the development of optimal dosing regimens. The goal of this research is to investigate the most significant factors affecting the rilpivirine release rate from PLGA-based ISFI, including the influence of the polymer content and structure (i.e. molecular weights, lactide/glycolide ratios, and chemistry of the terminal group). Thus, the use of a low molecular weight PLGA reduces the burst-effect from 17.5 to 4.7% of the drug content and enables continuous release of rilpivirine over the period of 42 days. At the same time, a more hydrophobic PLGA with a lactide/glycolide ratio of 75/25 and a terminal ester group affects to a greater extent the rilpivirine release rate in the third phase of the release process, when destruction of the polymer matrix starts. The rilpivirine release profile from the implant, formed by using a 30% (w/w) solution of PLGA with a higher content of lactic acid (75:25) and a terminal ester group, is similar to the dissolution profile of rilpivirine nanocrystals (Rekambis®).
Graphical abstract








Similar content being viewed by others
References
Flexner C (2019) Modern HIV therapy: progress and prospects. Clin Pharmacol Ther 105:61–70. https://doi.org/10.1002/cpt.1284
Williams J, Sayles HR, Meza JL et al (2013) Long-acting parenteral nanoformulated antiretroviral therapy: interest and attitudes of HIV-infected patients. Nanomedicine 8:1807–1813. https://doi.org/10.2217/nnm.12.214
Baert L, Kloostervan ’t G, Dries W et al (2009) Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur J Pharm Biopharm 72:502–508. https://doi.org/10.1016/j.ejpb.2009.03.006
Mordant C, Schmitt B, Pasquier E et al (2007) Synthesis of novel diarylpyrimidine analogues of TMC278 and their antiviral activity against HIV-1 wild-type and mutant strains. Eur J Med Chem 42:567–579. https://doi.org/10.1016/j.ejmech.2006.11.014
Namasivayam V, Vanangamudi M, Kramer VG et al (2019) The journey of HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) from lab to clinic. J Med Chem 62:4851–4883. https://doi.org/10.1021/acs.jmedchem.8b00843
(2021) FDA Approves cabenuva and vocabria for the treatment of HIV-1 infection. In: Food and Drug Administration. https://www.fda.gov/
Swindells S, Andrade-Villanueva J-F, Richmond GJ et al (2020) Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 382:1112–1123. https://doi.org/10.1056/nejmoa1904398
Spreen WR, Margolis DA, Pottage JC (2013) Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS 8:565–571. https://doi.org/10.1097/COH.0000000000000002
Gunawardana M, Remedios-Chan M, Miller CS et al (2015) Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrob Agents Chemother 59:3913–3919. https://doi.org/10.1128/AAC.00656-15
Solorio L, Olear AM, Hamilton JI et al (2012) Noninvasive characterization of the effect of varying PLGA molecular weight blends on in situ forming implant behavior using ultrasound imaging. Theranostics 2:1064–1077. https://doi.org/10.7150/thno.4181
Karunakaran D, Simpson SM, Su JT et al (2021) Design and testing of a cabotegravir implant for HIV prevention. J Controll Release 330:658–668. https://doi.org/10.1016/j.jconrel.2020.12.024
Barrett SE, Teller RS, Forster SP et al (2018) Extended-duration MK-8591-eluting implant as a candidate for HIV treatment and prevention. Antimicrob Agents Chemother 62:e01058-e1118
Kempe S, Mäder K (2012) In situ forming implants—an attractive formulation principle for parenteral depot formulations. J Controll Release 161:668–679. https://doi.org/10.1016/j.jconrel.2012.04.016
Chaudhary K, Patel MM, Mehta PJ (2019) Long-acting injectables: current perspectives and future promise. Crit Rev Ther Drug Carrier Syst 36:137–181. https://doi.org/10.1615/CritRevTherDrugCarrierSyst.2018025649
Maturavongsadit P, Shrivastava R, Sykes C et al (2021) Biodegradable polymeric solid implants for ultra-long-acting delivery of single or multiple antiretroviral drugs. Int J Pharm 605:120844. https://doi.org/10.1016/j.ijpharm.2021.120844
Benhabbour SR, Kovarova M, Jones C et al (2019) Ultra-long-acting tunable biodegradable and removable controlled release implants for drug delivery. Nat Commun 10:4324. https://doi.org/10.1038/s41467-019-12141-5
(2020) Assessment report. Rekambys
Thakur RRS, McMillan HL, Jones DS (2014) Solvent induced phase inversion-based in situ forming controlled release drug delivery implants. J Control Release 176:8–23. https://doi.org/10.1016/j.jconrel.2013.12.020
Zare M, Mobedi H, Barzin J et al (2008) Effect of additives on release profile of leuprolide acetate in an in situ forming controlled-release system. in vitro study. J Appl Polym Sci 107:3781–3787. https://doi.org/10.1002/app.27520
Ahmed TA, Ibrahim HM, Ibrahim F et al (2012) Development of biodegradable in situ implant and microparticle injectable formulations for sustained delivery of haloperidol. J Pharm Sci 101:3753–3762. https://doi.org/10.1002/jps.23250
Ibrahim TM, El-Megrab NA, El-Nahas HM (2020) Optimization of injectable PLGA in-situ forming implants of anti-psychotic risperidone via Box-Behnken design. J Drug Deliv Sci Technol 58:101803. https://doi.org/10.1016/j.jddst.2020.101803
Kanwar N, Sinha VR (2019) In situ forming depot as sustained-release drug delivery systems. Crit Rev Ther Drug Carrier Syst 36:93–136. https://doi.org/10.1615/CritRevTherDrugCarrierSyst.2018025013
Wang X, Burgess DJ (2021) Drug release from in situ forming implants and advances in release testing. Adv Drug Deliv Rev. https://doi.org/10.1016/j.addr.2021.113912
Luan X, Bodmeier R (2006) Influence of the poly(lactide-co-glycolide) type on the leuprolide release from in situ forming microparticle systems. J Controll Release 110:266–272. https://doi.org/10.1016/j.jconrel.2005.10.005
Chhabra S, Sachdeva V, Singh S (2007) Influence of end groups on in vitro release and biological activity of lysozyme from a phase-sensitive smart polymer-based in situ gel forming controlled release drug delivery system. Int J Pharm 342:72–77. https://doi.org/10.1016/j.ijpharm.2007.04.034
Patel V, Akbari B, Deshmukh A et al (2015) A review on PLGA based solvent induced in-situ forming implant. Res J Pharm Dos Technol 8:127
Romero GB, Keck CM, Müller RH (2016) Simple low-cost miniaturization approach for pharmaceutical nanocrystals production. Int J Pharm 501:236–244. https://doi.org/10.1016/j.ijpharm.2015.11.047
Ermolenko Y, Nazarova N, Belov A et al (2022) Potential of the capillary electrophoresis method for PLGA analysis in nano-sized drug formulations. J Drug Deliv Sci Technol 70:103220. https://doi.org/10.1016/j.jddst.2022.103220
Hatefi A, Amsden B (2002) Biodegradable injectable in situ forming drug delivery systems. J Control Release 80:9–28. https://doi.org/10.1016/S0168-3659(02)00008-1
Parent M, Nouvel C, Koerber M et al (2013) PLGA in situ implants formed by phase inversion: critical physicochemical parameters to modulate drug release. J Control Release 172:292–304. https://doi.org/10.1016/j.jconrel.2013.08.024
Williams PE, Crauwels HM, Basstanie ED (2015) Formulation and pharmacology of long-acting rilpivirine. Curr Opin HIV AIDS 10:233–238. https://doi.org/10.1097/COH.0000000000000164
Stokbroekx SCM (2010) Crystalline form of 4–4–4-(2-cyanoethenyl)-2,6-dimethylphenyl-amino-2-pyrimidinylaminobenzonitrile
Gad HA, El-Nabarawi MA, Abd El-Hady SS (2008) Formulation and evaluation of PLA and PLGA in situ implants containing secnidazole and/or doxycycline for treatment of periodontitis. AAPS Pharm Sci Tech 9:878. https://doi.org/10.1208/s12249-008-9126-9
Thackaberry EA, Wang X, Schweiger M et al (2014) Solvent-based formulations for intravenous mouse pharmacokinetic studies: tolerability and recommended solvent dose limits. Xenobiotica 44:235–241. https://doi.org/10.3109/00498254.2013.845706
Schwendeman SP, Shah RB, Bailey BA, Schwendeman AS (2014) Injectable controlled release depots for large molecules. J Controll Release 190:240–253. https://doi.org/10.1016/j.jconrel.2014.05.057
Bradshaw J, White S (2018) Combining human needs with high viscosity formulations. ONdrugDelivery Magazine 16–21
von Burkersroda F, Schedl L, Göpferich A (2002) Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials 23:4221–4231. https://doi.org/10.1016/S0142-9612(02)00170-9
Wischke C, Zhang Y, Mittal S, Schwendeman SP (2010) Development of PLGA-based injectable delivery systems for hydrophobic fenretinide. Pharm Res 27:2063–2074. https://doi.org/10.1007/s11095-010-0202-y
Ermolenko YV, Semyonkin AS, Ulianova YV et al (2020) Role of hydrolytic degradation of polylactide drug carriers in developing micro- and nanoscale polylactide-based drug dosage forms. Russ Chem Bull 69:1416–1427. https://doi.org/10.1007/s11172-020-2918-0
Astaneh R, Erfan M, Moghimi H, Mobedi H (2009) Changes in morphology of in situ forming PLGA implant prepared by different polymer molecular weight and its effect on release behavior. J Pharm Sci 98:135–145. https://doi.org/10.1002/jps.21415
Hopkins KA, Vike N, Li X et al (2019) Noninvasive characterization of in situ forming implant diffusivity using diffusion-weighted MRI. J Controll Release 309:289–301. https://doi.org/10.1016/j.jconrel.2019.07.019
Patel RB, Solorio L, Wu H et al (2010) Effect of injection site on in situ implant formation and drug release in vivo. J Controll Release 147:350–358. https://doi.org/10.1016/j.jconrel.2010.08.020
Graham PD, Brodbeck KJ, McHugh AJ (1999) Phase inversion dynamics of PLGA solutions related to drug delivery. J Controll Release 58:233–245. https://doi.org/10.1016/S0168-3659(98)00158-8
Solorio L, Babin BM, Patel RB et al (2010) Noninvasive characterization of in situ forming implants using diagnostic ultrasound. J Control Release 143:183–190. https://doi.org/10.1016/j.jconrel.2010.01.001
Joiner JB, Prasher A, Young IC et al (2022) Effects of drug physicochemical properties on in-situ forming implant polymer degradation and drug release kinetics. Pharmaceutics 14:1188. https://doi.org/10.3390/pharmaceutics14061188
Acknowledgements
The study was carried out with the financial support of the Russian Foundation for Basic Research within the framework of the scientific project No. 20-315-90104.The authors would like to thank Purac Biochem bv., Netherlands, for providing samples of Purasorb® PDLG 5004 and Purasorb® PDLG 5004A polymers. The authors are grateful to Dr. Alexander Kurkin (M.V. Lomonosov Moscow State University) for a generous gift of rilpivirine substance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ulianova, Y., Ermolenko, Y., Tkachenko, S. et al. Tuning the release rate of rilpivirine from PLGA-based in situ forming implants. Polym. Bull. 80, 11401–11420 (2023). https://doi.org/10.1007/s00289-022-04623-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04623-2