Abstract
In recent years, water purification by membrane desalination techniques has been growing drastically; after all, water scarcity is a significant issue to deal with in some parts of the world. To put one step forward toward resolving the issue of water scarcity, the best way is to upgrade the current desalination technique and membranes so that the output of clear water will be improved. In this review, we will focus on enhancing some crucial properties of the Polyamide (PA) and Polyimide (PI) membranes by incorporating some functional additives. Ag NPs (Silver nanoparticles), Cu NPs (Copper nanoparticles), GO (Graphene oxide), SWCNT (Single-walled carbon nanotube), and MWCNT (multi-walled carbon nanotube) are some of the additives which can be used with PA/PI active layer to improve some essential properties of membrane-like antifouling, biofouling, low water flux, selectivity, permeability, hydrophilicity, hydrophobicity, etc. The deposition of such additives onto the surface of the Polyimide/Polyamide coat or membrane can be done using interfacial polymerization or phase inversion. Membrane filtration can be done using reverse osmosis and electrodialysis techniques. A thin-film composite membrane comprising PA and MWCNTs, yielded a water flux of almost 25.9 L m−2 h−1, with a salt rejection of 98.1% exhibiting excellent hydrophilicity with a water contact angle of 59.6°.
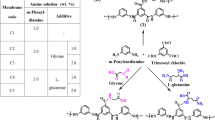
Graphical abstract






Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in the submitted manuscript.
Code availability
Not applicable.
Abbreviations
- aCN/AP:
-
Silver phosphate-loaded acidified graphitic carbon nitride
- Ag:
-
Silver
- Ag NPs:
-
Silver nanoparticles
- AgNO3 :
-
Silver nitrate
- CNT:
-
Carbon nanotube
- Cu:
-
Copper
- CuCl2·2H2O:
-
Dehydrated copper chloride
- CuNPs:
-
Copper nanoparticles
- DAPPC:
-
1,4-Bis(3-aminopropyl)-piperazine propane carboxylate
- ED:
-
Electrodialysis
- GO:
-
Graphene oxide
- GO NPs:
-
Graphene oxide nanoparticles
- GOQD:
-
Graphene oxide quantum dots
- IP:
-
Interfacial polymerization
- MED:
-
Multi-effect desalination
- MOF:
-
Metal organic framework
- MSF:
-
Multistage flash desalination
- MTFN:
-
Multifunctional thin-film nanocomposite
- MMM:
-
Mix matrix membrane
- MPD:
-
M-phenylene diamine
- MWCNT:
-
Muti-walled carbon nanotube
- Na2SO3 :
-
Sodium sulfite
- Na2SO4 :
-
Sodium sulfate
- NaCl:
-
Sodium chloride
- PA:
-
Polyamide
- PAN:
-
Polyacrylonitrile
- PEI:
-
Polyethyleneimine
- PES:
-
Polyethersulfone
- PI:
-
Polyimide
- PIP:
-
Piperazine
- PSF:
-
Polysulfone
- PVDF:
-
Polyvinylidene fluoride
- PVP:
-
Polyvinyl pyrrolidone
- RO:
-
Reverse osmosis
- SPI:
-
Sulfonated polyimide
- SWCNT:
-
Single-walled carbon nanotube
- T (\(^\circ{\rm C} )\) :
-
Temperature
- TFC:
-
Thin-film nanocomposite
- TiO2 :
-
Titanium dioxide
- TMC:
-
Trimesoyl chloride
- TNT:
-
Titania nanotubes
References
Dixit F, Zimmermann K, Dutta R et al (2022) Application of MXenes for water treatment and energy-efficient desalination: a review. J Hazard Mater 423:127050. https://doi.org/10.1016/j.jhazmat.2021.127050
Kavitha VU, Kandasubramanian B (2020) Tannins for wastewater treatment. SN Appl Sci 2:1081. https://doi.org/10.1007/s42452-020-2879-9
Wang D, Li J, Gao B et al (2021) Triple-layered thin film nanocomposite membrane toward enhanced forward osmosis performance. J Memb Sci 620:118879. https://doi.org/10.1016/j.memsci.2020.118879
Gupta P, Lapalikar V, Kundu R, Balasubramanian K (2016) Recent Advances in membrane based waste water treatment technology: a review. Energy Environ Focus 5:241–267. https://doi.org/10.1166/eef.2016.1227
Gude VG (2018) Energy storage for desalination. Renew Energy Powered Desalin Handb Appl Thermodyn. https://doi.org/10.1016/B978-0-12-815244-7.00010-6
Rastogi S, Kandasubramanian B (2020) Application of electrospun materials in water treatment. In: Electrospun materials and their allied applications. Wiley, pp 151–183
Turek M, Mitko K, Piotrowski K et al (2017) Prospects for high water recovery membrane desalination. Desalination 401:180–189. https://doi.org/10.1016/j.desal.2016.07.047
Sunada K, Kikuchi Y, Hashimoto K, Fujishima A (1998) Bactericidal and detoxification effects of TiO2 thin film photocatalysts. Environ Sci Technol 32:726–728. https://doi.org/10.1021/es970860o
Issac MN, Kandasubramanian B (2021) Effect of microplastics in water and aquatic systems. Environ Sci Pollut Res 28:19544–19562. https://doi.org/10.1007/s11356-021-13184-2
Lee KP, Arnot TC, Mattia D (2011) A review of reverse osmosis membrane materials for desalination: development to date and future potential. J Memb Sci 370:1–22. https://doi.org/10.1016/j.memsci.2010.12.036
Gore PM, Purushothaman A, Naebe M, et al (2019) Nanotechnology for oil-water separation, pp 299–339
Pillai A, Kandasubramanian B (2020) Carbon xerogels for effluent treatment. J Chem Eng Data 65:2255–2270. https://doi.org/10.1021/acs.jced.0c00092
Simon S, Balasubramanian K (2018) Facile immobilization of camphor soot on electrospun hydrophobic membrane for oil-water separation. Mater Focus 7:295–303. https://doi.org/10.1166/mat.2018.1511
Mayilswamy N, Kandasubramanian B (2022) Green composites prepared from soy protein, polylactic acid (PLA), starch, cellulose, chitin: a review. Emergent Mater 5:727–753. https://doi.org/10.1007/s42247-022-00354-2
Subash A, Naebe M, Wang X, Kandasubramanian B (2023) Biopolymer: a sustainable and efficacious material system for effluent removal. J Hazard Mater 443:130168. https://doi.org/10.1016/j.jhazmat.2022.130168
Mayilswamy N, Boney N, Kandasubramanian B (2022) Fabrication and molecular dynamics studies of layer-by-layer polyelectrolytic films. Eur Polym J 163:110945. https://doi.org/10.1016/j.eurpolymj.2021.110945
Sharma S, Balasubramanian K (2015) Molecularly imprinted and nanoengineered camphor soot functionalized PAN-nanofibers for effluent treatment. RSC Adv 5:31732–31741. https://doi.org/10.1039/c5ra02861b
Rule P, Balasubramanian K, Gonte RR (2014) Uranium(VI) remediation from aqueous environment using impregnated cellulose beads. J Environ Radioact 136:22–29. https://doi.org/10.1016/j.jenvrad.2014.05.004
Gore PM, Naebe M, Wang X, Kandasubramanian B (2020) Silk fibres exhibiting biodegradability and superhydrophobicity for recovery of petroleum oils from oily wastewater. J Hazard Mater 389:121823. https://doi.org/10.1016/j.jhazmat.2019.121823
Nitesh Singh NS, Balasubramanian K (2014) An effective technique for removal and recovery of uranium (VI) from aqueous solution using cellulose–camphor soot nanofibers. RSC Adv 4:27691–27701. https://doi.org/10.1039/C4RA01751J
Mishra P, Balasubramanian K (2014) Nanostructured microporous polymer composite imprinted with superhydrophobic camphor soot, for emphatic oil–water separation. RSC Adv 4:53291–53296. https://doi.org/10.1039/C4RA07410F
Gore P, Khraisheh M, Kandasubramanian B (2018) Nanofibers of resorcinol–formaldehyde for effective adsorption of As (III) ions from mimicked effluents. Environ Sci Pollut Res 25:11729–11745. https://doi.org/10.1007/s11356-018-1304-z
Gonte RR, Shelar G, Balasubramanian K (2014) Polymer–agro-waste composites for removal of Congo red dye from wastewater: adsorption isotherms and kinetics. Desalin Water Treat 52:7797–7811. https://doi.org/10.1080/19443994.2013.833876
Zaman NK, Rohani R, Mohammad AW, Isloor AM (2017) Polyimide-graphene oxide nanofiltration membrane: characterizations and application in enhanced high concentration salt removal. Chem Eng Sci. https://doi.org/10.1016/j.ces.2017.11.034
Noy A, Park HG, Fornasiero F et al (2007) Nanofluidics in carbon nanotubes. Nano Today 2:22–29. https://doi.org/10.1016/S1748-0132(07)70170-6
Okumus E, Gurkan T, Yilmaz L (1994) Development of a mixed-matrix membrane for pervaporation. Sep Sci Technol 29:2451–2473. https://doi.org/10.1080/01496399408002203
Ismail AF, Goh PS, Sanip SM, Aziz M (2009) Transport and separation properties of carbon nanotube-mixed matrix membrane. Sep Purif Technol 70:12–26. https://doi.org/10.1016/j.seppur.2009.09.002
Udayakumar KV, Gore PM, Kandasubramanian B (2021) Foamed materials for oil-water separation. Chem Eng J Adv 5:100076. https://doi.org/10.1016/j.ceja.2020.100076
Deoray N, Kandasubramanian B (2018) Review on three-dimensionally emulated fiber-embedded lactic acid polymer composites: opportunities in engineering sector. Polym Plast Technol Eng 57:860–874. https://doi.org/10.1080/03602559.2017.1354226
Patadiya J, Gawande A, Joshi G, Kandasubramanian B (2021) Additive manufacturing of shape memory polymer composites for futuristic technology. Ind Eng Chem Res 60:15885–15912. https://doi.org/10.1021/acs.iecr.1c03083
Khatavkar N, Balasubramanian K (2016) Composite materials for supersonic aircraft radomes with ameliorated radio frequency transmission-a review. RSC Adv 6:6709–6718. https://doi.org/10.1039/C5RA18712E
Wan C, Zhao F, Bao X et al (2008) Surface characteristics of polyhedral oligomeric silsesquioxane modified clay and its application in polymerization of macrocyclic polyester oligomers. J Phys Chem B 112:11915–11922. https://doi.org/10.1021/jp805259q
Sahoo BN, Kandasubramanian B (2014) An experimental design for the investigation of water repellent property of candle soot particles. Mater Chem Phys 148:134–142. https://doi.org/10.1016/j.matchemphys.2014.07.022
Mayilswamy N, Jaya Prakash N, Kandasubramanian B (2022) Design and fabrication of biodegradable electrospun nanofibers loaded with biocidal agents. Int J Polym Mater Polym Biomater. https://doi.org/10.1080/00914037.2021.2021905
Cadotte JE, Petersen RJ, Larson RE, Erickson EE (1980) A new thin-film composite seawater reverse osmosis membrane. Desalination 32:25–31. https://doi.org/10.1016/S0011-9164(00)86003-8
Cadotte JE (1985) Evolution of composite reverse osmosis membranes, pp 273–294
Freger V, Ramon GZ (2021) Polyamide desalination membranes: Formation, structure, and properties. Prog Polym Sci 122:101451. https://doi.org/10.1016/j.progpolymsci.2021.101451
Petersen RJ (1993) Composite reverse osmosis and nanofiltration membranes. J Memb Sci 83:81–150. https://doi.org/10.1016/0376-7388(93)80014-O
Morgan PW (2011) Interfacial polymerization. In: Encyclopedia of polymer science and technology. Wiley, Hoboken
Raaijmakers MJT, Benes NE (2016) Current trends in interfacial polymerization chemistry. Prog Polym Sci 63:86–142. https://doi.org/10.1016/j.progpolymsci.2016.06.004
Feldman D (2017) Polyamide nanocomposites. J Macromol Sci Part A 54:255–262. https://doi.org/10.1080/10601325.2017.1282700
Ben-Sasson M, Zodrow KR, Genggeng Q et al (2014) Surface functionalization of thin-film composite membranes with copper nanoparticles for antimicrobial surface properties. Environ Sci Technol 48:384–393. https://doi.org/10.1021/es404232s
Yu L, Zhou W, Li Y et al (2019) Antibacterial thin-film nanocomposite membranes incorporated with graphene oxide quantum dot-mediated silver nanoparticles for reverse osmosis application. ACS Sustain Chem Eng 7:8724–8734. https://doi.org/10.1021/acssuschemeng.9b00598
He M, Fan X, Yang Z et al (2016) Antifouling high-flux membranes via surface segregation and phase separation controlled by the synergy of hydrophobic and hydrogen bond interactions. J Memb Sci 520:814–822. https://doi.org/10.1016/j.memsci.2016.08.044
Yip NY, Tiraferri A, Phillip WA et al (2010) High performance thin-film composite forward osmosis membrane. Environ Sci Technol 44:3812–3818. https://doi.org/10.1021/es1002555
Qiu M, He C (2018) Novel zwitterion-silver nanocomposite modified thin-film composite forward osmosis membrane with simultaneous improved water flux and biofouling resistance property. Appl Surf Sci 455:492–501. https://doi.org/10.1016/j.apsusc.2018.06.020
Ma X-H, Yang Z, Yao Z-K et al (2017) A facile preparation of novel positively charged MOF/chitosan nanofiltration membranes. J Memb Sci 525:269–276. https://doi.org/10.1016/j.memsci.2016.11.015
Ben-Sasson M, Lu X, Bar-Zeev E et al (2014) In situ formation of silver nanoparticles on thin-film composite reverse osmosis membranes for biofouling mitigation. Water Res 62:260–270. https://doi.org/10.1016/j.watres.2014.05.049
Sharma L, Ye L, Yong C et al (2022) Aquaporin-based membranes made by interfacial polymerization in hollow fibers: visualization and role of aquaporin in water permeability. J Memb Sci 654:120551. https://doi.org/10.1016/j.memsci.2022.120551
Yang Z, Guo H, Yao Z et al (2019) Hydrophilic silver nanoparticles induce selective nanochannels in thin film nanocomposite polyamide membranes. Environ Sci Technol 53:5301–5308. https://doi.org/10.1021/acs.est.9b00473
Dong G, Hou J, Wang J et al (2016) Enhanced CO2/N2 separation by porous reduced graphene oxide/Pebax mixed matrix membranes. J Memb Sci 520:860–868. https://doi.org/10.1016/j.memsci.2016.08.059
Abadikhah H, Kalali EN, Behzadi S et al (2018) Amino functionalized silica nanoparticles incorporated thin film nanocomposite membrane with suppressed aggregation and high desalination performance. Polymer (Guildf) 154:200–209. https://doi.org/10.1016/j.polymer.2018.09.007
Abadikhah H, Naderi Kalali E, Khodi S et al (2019) Multifunctional thin-film nanofiltration membrane incorporated with reduced graphene oxide@TiO2@Ag nanocomposites for high desalination performance, dye retention, and antibacterial properties. ACS Appl Mater Interfaces 11:23535–23545. https://doi.org/10.1021/acsami.9b03557
Gonte RR, Deb PC, Balasubramanian K (2013) Hydrogen sorption onto hypercrosslinked polymer decorated with metal-organic framework. J Polym 2013:1–8. https://doi.org/10.1155/2013/684584
Zirehpour A, Rahimpour A, Ulbricht M (2017) Nano-sized metal organic framework to improve the structural properties and desalination performance of thin film composite forward osmosis membrane. J Memb Sci 531:59–67. https://doi.org/10.1016/j.memsci.2017.02.049
Wang Z, Wang Z, Lin S et al (2018) Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination. Nat Commun 9:2004. https://doi.org/10.1038/s41467-018-04467-3
Zhu Y, Xie W, Gao S et al (2016) Single-walled carbon nanotube film supported nanofiltration membrane with a nearly 10 nm thick polyamide selective layer for high-flux and high-rejection desalination. Small 12:5034–5041. https://doi.org/10.1002/smll.201601253
Chen X, Dai Y, Wang X et al (2015) Synthesis and characterization of Ag3PO4 immobilized with graphene oxide (GO) for enhanced photocatalytic activity and stability over 2,4-dichlorophenol under visible light irradiation. J Hazard Mater 292:9–18. https://doi.org/10.1016/j.jhazmat.2015.01.032
Yang X, Cai H, Bao M et al (2018) Insight into the highly efficient degradation of PAHs in water over graphene oxide/Ag3PO4 composites under visible light irradiation. Chem Eng J 334:355–376. https://doi.org/10.1016/j.cej.2017.09.104
Li S, Gao B, Wang Y et al (2019) Antibacterial thin film nanocomposite reverse osmosis membrane by doping silver phosphate loaded graphene oxide quantum dots in polyamide layer. Desalination 464:94–104. https://doi.org/10.1016/j.desal.2019.04.029
Ambekar RS, Kandasubramanian B (2020) Antimicrobial electrospun materials. In: Electrospun materials and their allied applications. Wiley, pp 483–514
Hirsch UM, Teuscher N, Rühl M, Heilmann A (2019) Plasma-enhanced magnetron sputtering of silver nanoparticles on reverse osmosis membranes for improved antifouling properties. Surf Interfaces 16:1–7. https://doi.org/10.1016/j.surfin.2019.04.003
Bagchi B, Dey S, Bhandary S et al (2012) Antimicrobial efficacy and biocompatibility study of copper nanoparticle adsorbed mullite aggregates. Mater Sci Eng C 32:1897–1905. https://doi.org/10.1016/j.msec.2012.05.011
Ren G, Hu D, Cheng EWC et al (2009) Characterisation of copper oxide nanoparticles for antimicrobial applications. Int J Antimicrob Agents 33:587–590. https://doi.org/10.1016/j.ijantimicag.2008.12.004
García A, Quintero Y, Vicencio N et al (2016) Influence of TiO 2 nanostructures on anti-adhesion and photoinduced bactericidal properties of thin film composite membranes. RSC Adv 6:82941–82948. https://doi.org/10.1039/C6RA17999A
García A, Rodríguez B, Oztürk D et al (2018) Incorporation of CuO nanoparticles into thin-film composite reverse osmosis membranes (TFC-RO) for antibiofouling properties. Polym Bull 75:2053–2069. https://doi.org/10.1007/s00289-017-2146-4
Zhang L, Wang H, Yu W et al (2012) Facile and large-scale synthesis of functional poly(m-phenylenediamine) nanoparticles by Cu2+-assisted method with superior ability for dye adsorption. J Mater Chem 22:18244. https://doi.org/10.1039/c2jm32859c
Balasubramanian K, Yadav R, Prajith P (2015) Antibacterial nanofibers of polyoxymethylene/gold for pro-hygiene applications. Int J Plast Technol 19:363–367. https://doi.org/10.1007/s12588-015-9127-y
Rodríguez B, Oztürk D, Rosales M et al (2018) Antibiofouling thin-film composite membranes (TFC) by in situ formation of Cu-(m-phenylenediamine) oligomer complex. J Mater Sci 53:6325–6338. https://doi.org/10.1007/s10853-018-2039-4
Ali MEA, Hassan FM, Feng X (2016) Improving the performance of TFC membranes via chelation and surface reaction: applications in water desalination. J Mater Chem A 4:6620–6629. https://doi.org/10.1039/C6TA01460G
Ramdayal BK (2014) Antibacterial application of polyvinylalcohol-nanogold composite membranes. Colloids Surf A Physicochem Eng Asp 455:174–178. https://doi.org/10.1016/j.colsurfa.2014.04.050
Issac MN, Kandasubramanian B (2020) Review of manufacturing three-dimensional-printed membranes for water treatment. Environ Sci Pollut Res 27:36091–36108. https://doi.org/10.1007/s11356-020-09452-2
Ma N, Wei J, Liao R, Tang CY (2012) Zeolite-polyamide thin film nanocomposite membranes: towards enhanced performance for forward osmosis. J Memb Sci 405–406:149–157. https://doi.org/10.1016/j.memsci.2012.03.002
Hu M, Mi B (2013) Enabling graphene oxide nanosheets as water separation membranes. Environ Sci Technol 47:3715–3723. https://doi.org/10.1021/es400571g
Eslah SS, Shokrollahzadeh S, Jazani OM (2017) Forward osmosis water desalination : Fabrication of graphene oxide-polyamide/polysulfone thin-film nanocomposite membrane with high water flux and low reverse salt diffusion. Sep Sci Technol. https://doi.org/10.1080/01496395.2017.1398261
Purabgola A, Mayilswamy N, Kandasubramanian B (2022) Graphene-based TiO2 composites for photocatalysis & environmental remediation: synthesis and progress. Environ Sci Pollut Res 29:32305–32325. https://doi.org/10.1007/s11356-022-18983-9
Zhao X, Tong Z, Liu X, et al (2020) Facile preparation of polyamide-graphene oxide composite membranes for upgrading pervaporation desalination performances of hypersaline solutions. https://doi.org/10.1021/acs.iecr.0c01417
Thakur K, Kandasubramanian B (2019) Graphene and graphene oxide-based composites for removal of organic pollutants: a review. J Chem Eng Data 64:833–867. https://doi.org/10.1021/acs.jced.8b01057
Kim I-C, Jeong B-R, Kim S-J, Lee K-H (2013) Preparation of high flux thin film composite polyamide membrane: the effect of alkyl phosphate additives during interfacial polymerization. Desalination 308:111–114. https://doi.org/10.1016/j.desal.2012.08.001
Bano S, Mahmood A, Kim S, Lee K (2014) membrane with improved fl ux and antifouling properties. https://doi.org/10.1039/C4TA03607G
Dong Y, Chen C, Zheng X et al (2012) One-step and high yield simultaneous preparation of single- and multi-layer graphene quantum dots from CX-72 carbon black. J Mater Chem 22:8764. https://doi.org/10.1039/c2jm30658a
Seyedpour SF, Rahimpour A, Shamsabadi AA, Soroush M (2018) Graphical abstract. Chem Eng Res Des. https://doi.org/10.1016/j.cherd.2018.09.041
Pang R, Zhang K (2017) RSC Advances A facile and viable approach to fabricate polyamide membranes functionalized with graphene oxide. RSC Adv 7:53463–53471. https://doi.org/10.1039/C7RA11358G
Shi J, Wu W, Xia Y et al (2018) Con fi ned interfacial polymerization of polyamide-graphene oxide composite membranes for water desalination Pure water MPD solution. Desalination 441:77–86. https://doi.org/10.1016/j.desal.2018.04.030
Inurria A, Cay-durgun P, Rice D et al (2018) Polyamide thin- fi lm nanocomposite membranes with graphene oxide nanosheets: balancing membrane performance and fouling propensity. Desalination. https://doi.org/10.1016/j.desal.2018.07.004
Abbaszadeh M, Krizak D, Kundu S (2019) Layer-by-layer assembly of graphene oxide nanoplatelets embedded desalination membranes with improved chlorine resistance. Desalination 470:114116. https://doi.org/10.1016/j.desal.2019.114116
Yi Z, Shao F, Yu L et al (2020) Chemical grafting N-GOQD of polyamide reverse osmosis membrane with improved chlorine resistance, water fl ux and NaCl rejection. Desalination 479:114341. https://doi.org/10.1016/j.desal.2020.114341
Sahoo BN, Balasubramanian K (2015) A nanocellular PVDF–graphite water-repellent composite coating. RSC Adv 5:6743–6751. https://doi.org/10.1039/C4RA06704E
Sun H, Li D, Liu B, Yao J (2019) Enhancing the permeability of TFC membranes based on incorporating polyamide matrix into MWCNTs framework. Appl Surf Sci 496:143680. https://doi.org/10.1016/j.apsusc.2019.143680
Taylor P, Park J, Choi W, et al. Desalination and water treatment enhancement of chlorine resistance in carbon nanotube based nanocomposite reverse osmosis membranes enhancement of chlorine resistance in carbon nanotube-based nanocomposite reverse osmosis membranes. 37–41. https://doi.org/10.5004/dwt.2010.1686
Azelee IW, Goh PS, Lau WJ et al (2017) Enhanced desalination of polyamide thin fi lm nanocomposite incorporated with acid treated multiwalled carbon nanotube-titania nanotube hybrid. Desalination 409:163–170. https://doi.org/10.1016/j.desal.2017.01.029
Farahbaksh J, Delnavaz M, Vatanpour V (2017) Investigation of raw and oxidized multiwalled carbon nanotubes in fabrication of reverse osmosis polyamide membranes for improvement in desalination and antifouling properties. Desalination 410:1–9. https://doi.org/10.1016/j.desal.2017.01.031
Park J, Choi W, Kim SH et al (2010) Enhancement of chlorine resistance in carbon nanotube based nanocomposite reverse osmosis membranes. Desalin Water Treat 15:198–204. https://doi.org/10.5004/dwt.2010.1686
Mahdavi MR, Delnavaz M, Vatanpour V (2017) Fabrication and water desalination performance of piperazine–polyamide nanocomposite nanofiltration membranes embedded with raw and oxidized MWCNTs. J Taiwan Inst Chem Eng 75:189–198. https://doi.org/10.1016/j.jtice.2017.03.039
Rashed AO, Esawi AMK, Ramadan AR (2020) Novel polysulfone/carbon nanotube-polyamide thin film nanocomposite membranes with improved water flux for forward osmosis desalination. ACS Omega 5:14427–14436. https://doi.org/10.1021/acsomega.0c00973
Zhao M, Zhang HF, Huang H, Zhang YS (2017) Preparation and properties of nanocomposite MWCNTs/polyamide reverse osmosis membrane for desalination by interfacial polymerization. Key Eng Mater 727:1016–1025. https://doi.org/10.4028/www.scientific.net/KEM.727.1016
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339–1339. https://doi.org/10.1021/ja01539a017
Huang A, Feng B (2018) Synthesis of novel graphene oxide-polyimide hollow fi ber membranes for seawater desalination. J Memb Sci 548:59–65. https://doi.org/10.1016/j.memsci.2017.11.016
Ba C, Langer J, Economy J (2009) Chemical modification of P84 copolyimide membranes by polyethylenimine for nanofiltration. 327:49–58. https://doi.org/10.1016/j.memsci.2008.10.051
Pandey RP, Shahi VK (2014) A N-o-sulphonic acid benzyl chitosan (NSBC) and N,N-dimethylene phosphonic acid propylsilane graphene oxide (NMPSGO) based multi-functional polymer electrolyte membrane with enhanced water retention and conductivity. RSC Adv 4:57200–57209. https://doi.org/10.1039/C4RA09581B
Tripathi BP, Chakrabarty T, Shahi VK (2010) Highly charged and stable cross-linked 4,4′-bis(4-aminophenoxy)biphenyl-3,3′-disulfonic acid (BAPBDS)-sulfonated poly(ether sulfone) polymer electrolyte membranes impervious to methanol. J Mater Chem 20:8036. https://doi.org/10.1039/c0jm01183e
Shukla G, Pandey RP, Shahi VK (2016) Author’s accepted manuscript improved performance. Elsevier
Wang S, Yi Z, Zhao X et al (2017) Aggregation suppressed thin film nanocomposite (TFN) membranes prepared with an in situ generation of TiO 2 nanoadditives. RSC Adv 7:26136–26144. https://doi.org/10.1039/C7RA02374J
Jeong B-H, Hoek EMV, Yan Y et al (2007) Interfacial polymerization of thin film nanocomposites: a new concept for reverse osmosis membranes. J Memb Sci 294:1–7. https://doi.org/10.1016/j.memsci.2007.02.025
Lee HS, Im SJ, Kim JH et al (2008) Polyamide thin-film nanofiltration membranes containing TiO2 nanoparticles. Desalination 219:48–56. https://doi.org/10.1016/j.desal.2007.06.003
Yang C, Xu W, Nan Y, Wang Y (2020) Novel solvent-resistant nanofiltration membranes using MPD co-crosslinked polyimide for efficient desalination. J Memb Sci 616:118603. https://doi.org/10.1016/j.memsci.2020.118603
Feng B, Xu K, Huang A (2016) RSC advances matrix membranes for desalination†. RSC Adv 7:2211–2217. https://doi.org/10.1039/C6RA24974D
Hamdy G, Taher A (2020) Enhanced chlorine-resistant and low biofouling reverse osmosis polyimide-graphene oxide thin film nanocomposite membranes for water desalination. Polym Eng Sci 60:2567–2580. https://doi.org/10.1002/pen.25495
Acknowledgements
The author would like to acknowledge Dr. S. P. Bhosle, Principal & Head, Maharashtra Institute of Technology, Aurangabad, Dr. Aniruddha Chatterjee, Head of Department, Plastic and polymer engineering, Maharashtra Institute of Technology, Aurangabad and Mrs. Suranjana Mandal, Associate Professor, Maharashtra Institute of Technology, Aurangabad and Dr. CP Ramanarayanan, Vice Chancellor of DIAT (DU), Pune, for their continuous encouragement and support. Authors wish to extend special thanks to Dr. Amrita Nighojkar, Miss. Niranjana Jaya Prakash, Mr. Jigar Patadiya, and Miss. Alsha Subash for their unwavering and continuous technical support throughout the review writing. The authors are thankful to the editors and anonymous reviewers who has helped in enhancing the quality of the manuscript.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Y A Ghodke contributed in Material preparation, data collection analysis and writing of the article. N Mayilswamy contributed to material preparation and writing of the article. B Kandasubramanian made substantial contribution to conceptualization, discussion and reviewed the manuscript before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
The authors consent to publish the article on acceptance.
Ethics approval
The submitted article complies with the ethical guidelines of the journal and does not contain the results of studies involving humans and/or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghodke, Y.A., Mayilswamy, N. & Kandasubramanian, B. Polyamide (PA)- and Polyimide (PI)-based membranes for desalination application. Polym. Bull. 80, 10661–10695 (2023). https://doi.org/10.1007/s00289-022-04559-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04559-7