Abstract
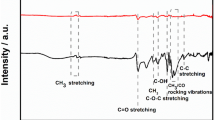
The ability to detect Cu2+ ions in aqueous media was investigated using poly(AA-co-NVIm-co-AAm) sensor hydrogels (RVH-1 to RVH-6) that have been synthesized by free radical polymerization. Synthesized hydrogels were characterized by FTIR, SEM–EDX, and TGA analysis. The swelling and rheological studies were performed to measure the maximum water retaining capacity of synthesized hydrogels and to understand the gel behavior. Among all the synthesized hydrogels, RVH-05 sensor hydrogel shows faster swelling with the highest swelling percentage (22,870%), which is responsible for the fast detection and enhanced adsorption of Cu2+ ions at a very low concentration. RVH-05 acts as a visual detector for Cu2+ ions by changing their color to blue with a limit of detection of 0.1 mg/L and also shows simultaneous removal capability with the enhanced adsorption of Cu2+ ions from their aqueous media. The effect of the removal conditions like contact time, pH, temperature, and initial metal ion concentration on the adsorption of Cu2+ ions has been tested and found maximum adsorption of 160 mg/g of Cu2+ ions. Adsorption isotherms were introduced and found Langmuir adsorption isotherm model best fitted for the chemical adsorption of the Cu2+ ions on the surface of RVH-05. RVH-05 shows fast detection, and effective adsorption of Cu2+ ions can reuse with high efficiency by simply soaking in a solution of disodium EDTA.
Graphical abstract
















Similar content being viewed by others
References
He C, Liu Z, Wu J et al (2021) Future global urban water scarcity and potential solutions. Nat Commun. https://doi.org/10.1038/s41467-021-25026-3
Khodadadi M, Malekpour A, Ansaritabar M (2017) Removal of Pb (II) and Cu (II) from aqueous solutions by NaA zeolite coated magnetic nanoparticles and optimization of method using experimental design. Microporous Mesoporous Mater 248:256–265. https://doi.org/10.1016/j.micromeso.2017.04.032
Saleh SM, Ali R, Hegazy MEF et al (2020) The natural compound chrysosplenol-D is a novel, ultrasensitive optical sensor for detection of Cu(II). J Mol Liq 302:112558. https://doi.org/10.1016/j.molliq.2020.112558
Bandmann O, Weiss KH, Kaler SG (2015) Wilson’s disease and other neurological copper disorders. Lancet Neurol 14:103–113. https://doi.org/10.1016/S1474-4422(14)70190-5
Hung YH, Bush AI, Cherny RA (2010) Copper in the brain and Alzheimer’s disease. J Biol Inorg Chem 15:61–76. https://doi.org/10.1007/s00775-009-0600-y
Rivera-Mancía S, Pérez-Neri I, Ríos C et al (2010) The transition metals copper and iron in neurodegenerative diseases. Chem Biol Interact 186:184–199. https://doi.org/10.1016/j.cbi.2010.04.010
Naat JN, Neolaka YAB, Lapailaka T et al (2021) Adsorption of Cu (II) and Pb (II) using silica@mercapto (hs@m) hybrid adsorbent synthesized from silica of Takari sand: optimization of parameters and kinetics. Rasayan J Chem 14:550–560. https://doi.org/10.31788/RJC.2021.1415803
Ozay H, Gungor Z, Yilmaz B et al (2020) Dual use of colorimetric sensor and selective copper removal from aqueous media with novel p (HEMA-co-TACYC) hydrogels: cyclen derivative as both monomer and crosslinker. J Hazard Mater 389:121848. https://doi.org/10.1016/j.jhazmat.2019.121848
Cheng Y, Li H, Fang C et al (2019) Facile synthesis of reduced graphene oxide/silver nanoparticles composites and their application for detecting heavy metal ions. J Alloy Compd 787:683–693. https://doi.org/10.1016/j.jallcom.2019.01.320
Aydin FA, Soylak M (2010) Separation, preconcentration and inductively coupled plasma-mass spectrometric (ICP-MS) determination of thorium(IV), titanium(IV), iron(III), lead(II) and chromium(III) on 2-nitroso-1-naphthol impregnated MCI GEL CHP20P resin. J Hazard Mater 173:669–674. https://doi.org/10.1016/j.jhazmat.2009.08.137
Ghaedi M, Shokrollahi A, Niknam K, Soylak M (2009) Cloud point extraction of copper, zinc, iron and nickel in biological and environmental samples by flame atomic absorption spectrometry. Sep Sci Technol 44:773–786. https://doi.org/10.1080/01496390802437164
Heilmann J, Boulyga SF, Heumann KG (2009) Development of an isotope dilution laser ablation ICP-MS method for multi-element determination in crude and fuel oil samples. J Anal At Spectrom 24:385–390. https://doi.org/10.1039/b819879a
Soylak M, Unsal YE (2010) Chromium and iron determinations in food and herbal plant samples by atomic absorption spectrometry after solid phase extraction on single-walled carbon nanotubes (SWCNTs) disk. Food Chem Toxicol 48:1511–1515. https://doi.org/10.1016/j.fct.2010.03.017
Darmokoesoemo H, Magdhalena PTWLC, Kusuma HS (2016) Telescope snail (Telescopium sp) and mangrove crab (Scylla sp) as adsorbent for the removal of Pb2+ from aqueous solutions. Rasayan J Chem 9:680–685
Ansari S, Ahmed N, Bux Mahar R et al (2021) Fabrication and characterization of electrospun zein/nylon-6 (ZN6) nanofiber membrane for hexavalent chromium removal. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-15729-x
Choudhury N, Mete S, Kambalapalli S, De P (2018) Side-chain glycylglycine-based polymer for simultaneous sensing and removal of copper (II) from aqueous medium. J Polym Sci A: Polym Chem 56:914–921. https://doi.org/10.1002/pola.28968
Qiu X, Li N, Yang S et al (2015) A new magnetic nanocomposite for selective detection and removal of trace copper ions from water. J Mater Chem A 3:1265–1271. https://doi.org/10.1039/c4ta05452k
Awual MR, Ismael M, Yaita T et al (2013) Trace copper (II) ions detection and removal from water using novel ligand modified composite adsorbent. Chem Eng J 222:67–76. https://doi.org/10.1016/j.cej.2013.02.042
Li M, Liu Z, Wang S et al (2018) Fluorescence detection and removal of copper from water using a biobased and biodegradable 2D soft material. Chem Commun 54:184–187. https://doi.org/10.1039/c7cc08035b
Liu YY, Fan XD (2002) Synthesis and characterization of pH- and temperature-sensitive hydrogel of N-isopropylacrylamide/cyclodextrin based copolymer. Polymer 43:4997–5003. https://doi.org/10.1016/S0032-3861(02)00350-6
Wang W, Wang J, Zhao Y et al (2020) High-performance two-dimensional montmorillonite supported-poly (acrylamide-co-acrylic acid) hydrogel for dye removal. Environ Pollut 257:113574. https://doi.org/10.1016/j.envpol.2019.113574
Mohammadzadeh Pakdel P, Peighambardoust SJ (2018) A review on acrylic based hydrogels and their applications in wastewater treatment. J Environ Manage 217:123–143. https://doi.org/10.1016/j.jenvman.2018.03.076
Li SN, Li B, Yu ZR et al (2020) Constructing dual ionically cross-linked poly(acrylamide-co-acrylic acid)/chitosan hydrogel materials embedded with chitosan decorated halloysite nanotubes for exceptional mechanical performance. Compos B Eng 194:108046. https://doi.org/10.1016/j.compositesb.2020.108046
Pekel N, Savaş H, Güven O (2002) Complex formation and adsorption of V3+, Cr3+ and Fe3+ ions with poly (n-vinylimidazoel). Colloid Polym Sci 280:46–51. https://doi.org/10.1007/s003960200006
Pekel N, Salih B, Güven O (2003) Activity studies of glucose oxidase immobilized onto poly(N-vinylimidazole) and metal ion-chelated poly(N-vinylimidazole) hydrogels. J Mol Catal B Enzym 21:273–282. https://doi.org/10.1016/S1381-1177(02)00232-1
Hezarkhani M, Yilmaz E (2019) Pullulan modification via poly(N-vinylimidazole) grafting. Int J Biol Macromol 123:149–156. https://doi.org/10.1016/j.ijbiomac.2018.11.022
Jiao C, Zhang J, Liu T et al (2020) Mechanically strong, tough, and shape deformable poly (acrylamide-co-vinylimidazole) hydrogels based on Cu2+ complexation. ACS Appl Mater Interfaces 12:44205–44214. https://doi.org/10.1021/acsami.0c13654
Sargsyan SH, Sargsyan TS, Khizantsyan KM et al (2020) Electrosynthesis of 1-vinylimidazole- and acrylic-acid-based metal-containing nanocomposites. Russ J Electrochem 56:87–91. https://doi.org/10.1134/S1023193520010073
Molina MJ, Gómez-Antón MR, Piérola IF (2007) Determination of the parameters controlling swelling of chemically cross-linked pH-sensitive poly(N-vinylimidazole) hydrogels. J Phys Chem B 111:12066–12074. https://doi.org/10.1021/jp074385k
Tirtom VN, Dinçer A (2021) Effective removal of heavy metals from an aqueous solution with poly(N-vinylimidazole-acrylamide) hydrogels. Sep Sci Technol 56:912–924. https://doi.org/10.1080/01496395.2020.1735434
Molina MJ, Gómez-Antón MR, Piérola IF (2002) pH-dependence of the swelling capacity of poly(N-vinylimidazole) hydrogels. Macromol Chem Phys 203:2075–2082. https://doi.org/10.1002/1521-3935(200210)203:14%3c2075::AID-MACP2075%3e3.0.CO;2-D
Molina MJ, Gómez‐Antón MR, Rivas BL, Maturana HA, Pierola IF (2001) Removal of Hg (II) from acid aqueous solutions by poly (N-Vinylimidazole) hydrogel. J Appl Polym Sci 79(8):1467–1475. https://doi.org/10.1002/1097-4628(20010222)79:83.0.CO;2-9
Zhong T, Feng X, Sun L et al (2021) Highly effective adsorption of copper ions by poly(vinyl imidazole) cryogels. Polym Bull 78:5873–5890. https://doi.org/10.1007/s00289-020-03413-y
Ayub A, Irfan A, Raza ZA et al (2021) Development of poly(1-vinylimidazole)-chitosan composite sorbent under microwave irradiation for enhanced uptake of Cd (II) ions from aqueous media. Polym Bull 79:807–827
Dadhaniya PV, Patel MP, Patel RG (2006) Swelling and dye adsorption study of novel superswelling [Acrylamide/N-vinylpyrrolidone/3(2-hydroxyethyl carbamoyl) acrylic acid] hydrogels. Polym Bull 57:21–31. https://doi.org/10.1007/s00289-006-0531-5
Mahida VP, Patel MP (2014) Synthesis of new superabsorbent poly (NIPAAm/AA/N-allylisatin) nanohydrogel for effective removal of As (V) and Cd(II) toxic metal ions. Chin Chem Lett 25:601–604. https://doi.org/10.1016/j.cclet.2014.01.031
Zhong T, Feng X, Sun L et al (2020) Highly effective adsorption of copper ions by poly(vinyl imidazole) cryogels. Polym Bull. https://doi.org/10.1007/s00289-020-03413-y
Gor AH, Dave PN (2020) Adsorptive abatement of ciprofloxacin using NiFe2O4 nanoparticles incorporated into G. ghatti-cl-P(AAm) nanocomposites hydrogel: isotherm, kinetic, and thermodynamic studies. Polym Bull 77:5589–5613. https://doi.org/10.1007/s00289-019-03032-2
El-Ghazali S, Kobayashi H, Khatri M et al (2021) Preparation of a cage-type polyglycolic acid/collagen nanofiber blend with improved surface wettability and handling properties for potential biomedical applications. Polymers. https://doi.org/10.3390/polym
Liu X, Wei Q (2016) Removal of methylene blue from aqueous solution using porous starch-: G -poly(acrylic acid) superadsorbents. RSC Adv 6:79853–79858. https://doi.org/10.1039/c6ra14903k
Jana S, Ray J, Mondal B et al (2018) pH responsive adsorption/desorption studies of organic dyes from their aqueous solutions by katira gum-cl-poly(acrylic acid-co-N-vinyl imidazole) hydrogel. Colloids Surf A 553:472–486. https://doi.org/10.1016/j.colsurfa.2018.06.001
Zhou Q, Lin X, Li B, Luo X (2014) Fluoride adsorption from aqueous solution by aluminum alginate particles prepared via electrostatic spinning device. Chem Eng J 256:306–315. https://doi.org/10.1016/j.cej.2014.06.101
Dadhaniya PV, Patel MP, Patel RG (2007) Copper and nickel removal from aqueous solutions using new chelating poly[acrylamide/N-vinyl pyrrolidone/3-(2-hydroxyethyl carbamoyl)acrylic acid] hydrogels. J Macromol Sci Part A Pure Appl Chem 44:769–777. https://doi.org/10.1080/10601320701353694
Özkahraman B, Acar I, Emik S (2011) Removal of cationic dyes from aqueous solutions with poly (N-isopropylacrylamide-co-itaconic acid) hydrogels. Polym Bull 66:551–570. https://doi.org/10.1007/s00289-010-0371-1
Patel YN, Patel MP (2013) Adsorption of azo dyes from water by new poly (3-acrylamidopropyl)- trimethylammonium chloride-co-N, N-dimethylacrylamide superabsorbent hydrogel—Equilibrium and kinetic studies. J Environ Chem Eng 1:1368–1374. https://doi.org/10.1016/j.jece.2013.09.024
Qu W, He D, Guo Y et al (2019) Characterization of modified Alternanthera philoxeroides by diethylenetriamine and its application in the adsorption of copper(II) ions in aqueous solution. Environ Sci Pollut Res 26:21189–21200. https://doi.org/10.1007/s11356-019-05472-9
Acknowledgements
This research was supported by the Department of Chemistry, Sardar Patel University. Authors are grateful to UGC, New Delhi, for the UGC-CPEPA Phase-II program sponsored under award letter no. F. No. 1–14/2002-2016(NS/PE) dated April 28, 2016, for assistance in person as well as UGC-CAS, Phase-II program sponsored under award letter no. F-540/5/CASII/2018 (SAP-I) dated July 25, 2018, for assistance in general.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare they have no financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hingrajiya, R.D., Kalola, A.G. & Patel, M.P. Poly(AA-co-NVIm-co-AAm) sensor hydrogels for the simultaneous visual detection and removal of Cu2+ ions from aqueous media. Polym. Bull. 80, 10099–10124 (2023). https://doi.org/10.1007/s00289-022-04544-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04544-0