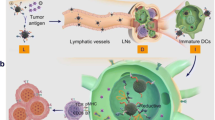
Abstract
An immune system responds to pathogens, toxic compounds, or certain physiological conditions which lead to inflammation. However, uncontrolled and excessive inflammation is associated with a variety of severe chronic conditions including intestinal disorders, cancer, diabetes, and myocardial infarction. The development of anti-inflammatory therapies to treat and manage relevant chronic diseases has resulted from a better understanding of inflammation. However, clinical outcomes vary among patients and serious adverse effects are often observed. Furthermore, clinical anti-inflammatory therapeutics have some limitations due to their insolubility in water, low bioavailability, and poor accessibility to subcellular compartments. To address these challenges, the drug delivery system specific to inflammation offers significant potential. A hydrogel is attractive as a drug delivery platform because of its outstanding characteristics, including swellability, biocompatibility, controlled degradation, and sustained drug release. Hydrogels have been widely used in biomedical applications for several reasons, using diverse polymers of synthetic and natural origin. The design of hydrogels relies heavily on proteins and peptides because proteins are the fundamental macromolecules in living organisms for biochemical, mechanical, and structural functions. Therefore, they provide us with a wide range of structural building blocks for the formation of various types of biomaterials, including hydrogels. Since natural proteins and peptides are biocompatible and biodegradable, they have features advantageous for their use as the building blocks of hydrogels for biomedical applications. This review aims to focus on hydrogels derived from protein and peptide-based systems and highlights recent trends in the use of protein and peptide-based hydrogels as drug delivery systems for inflammation.








Similar content being viewed by others
Data availability statement
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also form part of our future research study.
References
Chovatiya R, Medzhitov R (2014) Stress, inflammation, and defense of homeostasis. Mol Cell 54(2):281–288. https://doi.org/10.1016/j.molcel.2014.03.030
Zhou Y, Hong Y, Huang H (2016) Triptolide Attenuates inflammatory response in membranous glomerulo-nephritis rat via downregulation of NF-κB signaling pathway. Kidney Blood Press Res 41(6):901–910. https://doi.org/10.1159/000452591
Li J, Mooney DJ (2016) Designing hydrogels for controlled drug delivery. Nat Rev Mater 1(12). 10.1038/natrevmats.2016.71
Bahram M, Mohseni N, Moghtader M (2016) An introduction to hydrogels and some recent applications. In: Majee SB (eds) Emerging concepts in analysis and applications of hydrogels.. Rijeka: IntechOpen. https://doi.org/10.5772/64301
Hoare TR, Kohane DS (2008)Hydrogels in drug delivery: progress and challenges. Polymer 49(8):1993–2007. Available from: https://www.sciencedirect.com/science/article/pii/S0032386108000487
Jacob S, Nair AB, Shah J, Sreeharsha N, Gupta S, Shinu P (2021) Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics13(3). Available from: https://doi.org/10.3390/pharmaceutics13030357
Basso J, Miranda A, Nunes S, Cova T, Sousa J, Vitorino C, et al. (2018) Hydrogel-based drug delivery nanosystems for the treatment of brain tumors. Gels4(3). Available from: https://doi.org/10.3390/gels4030062
Caló E, Khutoryanskiy VV (2015) Biomedical applications of hydrogels: A review of patents and commercial products. Eur Polym J65:252–267. Available from: https://www.sciencedirect.com/science/article/pii/S0014305714004091
Corrente F, Matricardi P, Paolicelli P, Tita B, Vitali F, Casadei MA (2009) Physical carboxymethylscleroglucan/calcium ion hydrogels as modified drug delivery systems in topical formulations. Molecules 14(8):2684–2698.https://doi.org/10.3390/molecules14082684
Wichterle O, Lím D (1960) Hydrophilic gels for biological use. Nature 185(4706):117–118. Available from: https://www.nature.com/articles/185117a0
Jonker AM, Löwik D (2012) Peptide-and protein-based hydrogels. Chemistry. Available from: https://pubs.acs.org/doi/abs/https://doi.org/10.1021/cm202640w
Kopecek J (2007) Hydrogel biomaterials: a smart future? Biomaterials 28(34):5185–5192. https://doi.org/10.1016/j.biomaterials.2007.07.044
Katyal P, Mahmoudinobar F, Montclare JK (2020) Recent trends in peptide and protein-based hydrogels. Curr Opin Struct Biol 63:97–105. https://doi.org/10.1016/j.sbi.2020.04.007
Desai S, Harrison B (2010) Direct-writing of biomedia for drug delivery and tissue regeneration. In: Narayan R, Boland T, Lee YS (eds) Printed biomaterials: novel processing and modeling techniques for medicine and surgery. Springer, New York, p 71–89. https://doi.org/10.1007/978-1-4419-1395-1_5
Wu Y, Antony S, Meitzler JL, Doroshow JH (2014) Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett 345(2):164–173. https://doi.org/10.1016/j.canlet.2013.08.014
Tabas I, Glass CK (2013) Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 339(6116):166–172. Available from: https://science.sciencemag.org/content/339/6116/166.abstract
Calder PC, Albers R, Antoine JM, Blum S, Bourdet-Sicard R, Ferns GA et al (2009) Inflammatory disease processes and interactions with nutrition. Br J Nutr101(Suppl 1):S1–45. https://doi.org/10.1017/S0007114509377867
Deng Z, Liu S (2021) Inflammation-responsive delivery systems for the treatment of chronic inflammatory diseases. Drug Deliv Transl Res. https://doi.org/10.1007/s13346-021-00977-8
Frith JE, Cameron AR, Menzies DJ, Ghosh P, Whitehead DL, Gronthos S et al (2013) An injectable hydrogel incorporating mesenchymal precursor cells and pentosan polysulphate for intervertebral disc regeneration. Biomaterials. 34:9430–40. https://doi.org/10.1016/j.biomaterials.2013.08.072
Seo BB, Koh JT, Song SC (2017) Tuning physical properties and BMP-2 release rates of injectable hydrogel systems for an optimal bone regeneration effect. Biomaterials 122:91–104. https://doi.org/10.1016/j.biomaterials.2017.01.016
Tang G, Zhou B, Li F, Wang W, Liu Y, Wang X et al. (2020) Advances of naturally derived and synthetic hydrogels for intervertebral disk regeneration. Front Bioeng Biotechnol 8:745. https://doi.org/10.3389/fbioe.2020.00745
Brovold M, Almeida JI, Pla-Palacín I, Sainz-Arnal P, Sánchez-Romero N, Rivas JJ et al (2018) Naturally-derived biomaterials for tissue engineering applications. Adv Exp Med Biol 1077:421–449. https://doi.org/10.1007/978-981-13-0947-2_23
Ahmed EM (2015) Hydrogel: preparation, characterization, and applications: a review. J Advert Res 6(2):105–121. https://doi.org/10.1016/j.jare.2013.07.006
Kumar AC, Erothu H (2016) Synthetic polymer hydrogels. Biomedical applications of polymeric. Available from: https://books.google.com/books?hl=en&lr=&id=YeIqDQAAQBAJ&oi=fnd&pg=PA141&dq=Kumar+A+C+Erothu+H+(2016)+Synthetic+Polymer+Hydrogels+Biomedical+Applications+of+Polymeric+Materials+and+Composites+141+162&ots=wCSmBQUmR5&sig=vFHpF4g6vXrGOjIQfPXQRJoU2Ns
El-Sherbiny IM, Yacoub MH (2013) Hydrogel scaffolds for tissue engineering: progress and challenges. Glob Cardiol Sci Pract 2013(3):316–342. Available from: https://doi.org/10.5339/gcsp.2013.38
Singhal R, Gupta K (2016) A review: tailor-made hydrogel structures (classifications and synthesis parameters). Polym Plast Technol Eng 55(1):54–70. https://doi.org/10.1080/03602559.2015.1050520
Chang C, Zhang L (2011) Cellulose-based hydrogels: present status and application prospects. Carbohydr Polym 84(1):40–53. Available from: https://www.sciencedirect.com/science/article/pii/S0144861710010003
Ahmadi F, Oveisi Z, Samani SM, Amoozgar Z (2015) Chitosan based hydrogels: characteristics and pharmaceutical applications. Res Pharm Sci 10(1):1–16. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26430453
Catoira MC, Fusaro L, Di Francesco D, Ramella M, Boccafoschi F (2019) Overview of natural hydrogels for regenerative medicine applications. J Mater Sci Mater Med 30(10):115. https://doi.org/10.1007/s10856-019-6318-7
Fernández-Pérez J, Ahearne M (2019) The impact of decellularization methods on extracellular matrix derived hydrogels. Sci Rep 9(1):14933. https://doi.org/10.1038/s41598-019-49575-2
Freytes DO, Martin J, Velankar SS, Lee AS, Badylak SF (2008) Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials 29(11):1630–1637. https://doi.org/10.1016/j.biomaterials.2007.12.014
Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL (2009) Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials 30(29):5409–5416. https://doi.org/10.1016/j.biomaterials.2009.06.045
Travascio F (2016) Composition and function of the extracellular matrix in the human body. BoD—Books on Demand. Available from: https://play.google.com/store/books/details?id=bJ2RDwAAQBAJ
Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, et al () Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci Transl Med [Internet]. 2013 Feb 20;5(173):173ra25. Available from: https://doi.org/10.1126/scitranslmed.3005503
Wassenaar JW, Gaetani R, Garcia JJ, Braden RL, Luo CG, Huang D, et al (2016) Evidence for mechanisms underlying the functional benefits of a myocardial matrix hydrogel for post-MI treatment. J Am Coll Cardiol 67(9):1074–1086. https://doi.org/10.1016/j.jacc.2015.12.035
Saldin LT, Cramer MC, Velankar SS, White LJ, Badylak SF (2017) Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater 49:1–15. https://doi.org/10.1016/j.actbio.2016.11.068
Gagner JE, Kim W, Chaikof EL (2014) Designing protein-based biomaterials for medical applications. Acta Biomater 10(4):1542–57. Available from: https://www.sciencedirect.com/science/article/pii/S1742706113005072
Krishna OD, Kiick KL (2010) Protein- and peptide-modified synthetic polymeric biomaterials. Biopolymers 94(1):32–48. https://doi.org/10.1002/bip.21333
Sengupta D, Heilshorn SC (2010) Protein-engineered biomaterials: highly tunable tissue engineering scaffolds. Tissue Eng Part B Rev 16(3):285–93. https://doi.org/10.1089/ten.teb.2009.0591
Wang X, Kim HJ, Wong C, Vepari C, Matsumoto A, Kaplan DL (2006) Fibrous proteins and tissue engineering. Materials Today 9:44–53. Available from: https://doi.org/10.1016/s1369-7021(06)71742-4
Karsdal MA, Nielsen MJ, Sand JM, Henriksen K, Genovese F, Bay-Jensen AC et al (2013) Extracellular matrix remodeling: the common denominator in connective tissue diseases possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure vol 11, ASSAY and drug development technologies, pp 70–92. https://doi.org/10.1089/adt.2012.474
Heymer A, Haddad D, Weber M, Gbureck U, Jakob PM, Eulert J et al (2008) Iron oxide labelling of human mesenchymal stem cells in collagen hydrogels for articular cartilage repair. Biomaterials 29(10):1473–1483. https://doi.org/10.1016/j.biomaterials.2007.12.003
Phillips JB (2014) Building stable anisotropic tissues using cellular collagen gels. Organogenesis 10(1):6–8. https://doi.org/10.4161/org.27487
Plant AL, Bhadriraju K, Spurlin TA, Elliott JT (2009) Cell response to matrix mechanics: focus on collagen. Biochim Biophys Acta 1793(5):893–902. https://doi.org/10.1016/j.bbamcr.2008.10.012
Placzek M (2008) Tissue recombinations in collagen gels. Methods Mol Biol 461:325–35. https://doi.org/10.1007/978-1-60327-483-8_23
Patel ZS, Ueda H, Yamamoto M, Tabata Y, Mikos AG (2008) In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharm Res 25(10):2370–2378. https://doi.org/10.1007/s11095-008-9685-1
Kimura Y, Tabata Y (2010) Controlled release of stromal-cell-derived factor-1 from gelatin hydrogels enhances angiogenesis. J Biomater Sci Polym Ed 21(1):37–51. https://doi.org/10.1163/156856209X410193
Konishi M, Tabata Y, Kariya M, Suzuki A, Mandai M, Nanbu K et al (2003) In vivo anti-tumor effect through the controlled release of cisplatin from biodegradable gelatin hydrogel. J Control Release 92(3):301–313. https://doi.org/10.1016/s0168-3659(03)00364-x
Rattanaruengsrikul V, Pimpha N, Supaphol P (2012) In vitro efficacy and toxicology evaluation of silver nanoparticle-loaded gelatin hydrogel pads as antibacterial wound dressings. J Appl Polym Sci 124:1668–82. https://doi.org/10.1002/app.35195
Stevens KR, Einerson NJ, Burmania JA, Kao WJ (2002) In vivo biocompatibility of gelatin-based hydrogels and interpenetrating networks. J Biomater Sci Polym Ed 13(12):1353–1366. https://doi.org/10.1163/15685620260449741
Yamamoto M, Ikada Y, Tabata Y (2001) Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci Polym Ed 12(1):77–88. https://doi.org/10.1163/156856201744461
Asmani MN, Ai J, Amoabediny G, Noroozi A, Azami M, Ebrahimi-Barough S et al. (2013) Three-dimensional culture of differentiated endometrial stromal cells to oligodendrocyte progenitor cells (OPCs) in fibrin hydrogel. Cell Biol Int 37(12):1340–9. https://doi.org/10.1002/cbin.10171
des Rieux A, Shikanov A, Shea LD (2009) Fibrin hydrogels for non-viral vector delivery in vitro. J Control Release 136(2):148–54. Available from: https://www.sciencedirect.com/science/article/pii/S0168365909000935
Gamboa-Martínez TC, Luque-Guillén V, González-García C, Ribelles JLG, Gallego-Ferrer G (2015) Crosslinked fibrin gels for tissue engineering: two approaches to improve their properties. J Biomed Mater Res Part A. 103:614–621. https://doi.org/10.1002/jbm.a.35210
Guthold M, Liu W, Sparks EA, Jawerth LM, Peng L, Falvo M, et al (2007) A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers. Cell Biochem Biophys 49(3):165–181. https://doi.org/10.1007/s12013-007-9001-4
Lei P, Padmashali RM, Andreadis ST (2009) Cell-controlled and spatially arrayed gene delivery from fibrin hydrogels. Biomaterials 30(22):3790–3799. https://doi.org/10.1016/j.biomaterials.2009.03.049
Fathi A, Mithieux SM, Wei H, Chrzanowski W, Valtchev P, Weiss AS et al (2014) Elastin based cell-laden injectable hydrogels with tunable gelation, mechanical and biodegradation properties. Biomaterials 35(21):5425–5435. https://doi.org/10.1016/j.biomaterials.2014.03.026
Lim DW, Nettles DL, Setton LA, Chilkoti A (2008) In situ cross-linking of elastin-like polypeptide block copolymers for tissue repair. Biomacromolecules 9(1):222–230. https://doi.org/10.1021/bm7007982
McHale MK, Setton LA, Chilkoti A (2005) Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng 11(11–12):1768–1779. https://doi.org/10.1089/ten.2005.11.1768
Tomita N, Morita Y, Hattori K, Harada Y (2003) Culture of chondrocytes in fibroin–hydrogel sponge and engineering. Available from: https://content.iospress.com/articles/bio-medical-materials-and-engineering/bme264
Bose S, Bandyopadhyay A (2016) Materials and devices for bone disorders. Academic Press. Available from: https://play.google.com/store/books/details?id=8U7pCAAAQBAJ
Singh TRR, Laverty G, Donnelly R (2018) Hydrogels: design, synthesis and application in drug delivery and regenerative medicine. CRC Press. Available from: https://play.google.com/store/books/details?id=NGpQDwAAQBAJ
Fini M, Motta A, Torricelli P, Giavaresi G, Nicoli Aldini N, Tschon M et al (2005) The healing of confined critical size cancellous defects in the presence of silk fibroin hydroge. Biomaterials. 26: 3527–36. https://doi.org/10.1016/j.biomaterials.2004.09.040
Eissa AS, Khan SA (2005) Acid-induced Gelation of enzymatically modified, preheated whey proteins. J Agric Food Chem 53: 5010–7. https://doi.org/10.1021/jf047957w
Gunasekaran S, Ko S, Xiao L (2007) Use of whey proteins for encapsulation and controlled delivery applications. J Food Eng 83:31–40. https://doi.org/10.1016/j.jfoodeng.2006.11.001
Ustunol Z (2014) Applied food protein chemistry. John Wiley & Sons. Available from: https://play.google.com/store/books/details?id=haNsDwAAQBAJ
Prasad PN (2012) Frontiers of polymers and advanced materials. Springer US. Available from: https://play.google.com/store/books/details?id=9w-pmQEACAAJ
Reddy N, Aramwit P (2021) Sustainable uses of byproducts from silk processing. John Wiley & Sons. Available from: https://play.google.com/store/books/details?id=HXUrEAAAQBAJ
Wang Z, Zhang Y, Zhang J, Huang L, Liu J, Li Y, et al (2015) Exploring natural silk protein sericin for regenerative medicine: an injectable, photoluminescent, cell-adhesive 3D hydrogel. Sci Rep , 4. https://doi.org/10.1038/srep07064
Liu C, Zhang Q, Zhu S, Liu H, Chen J (2019) Preparation and applications of peptide-based injectable hydrogels. RSC Adv 9(48):28299–282311. Available from: https://pubs.rsc.org/fa/content/articlehtml/2019/ra/c9ra05934b
Zhou J, Li J, Du X, Xu B (2017) Supramolecular biofunctional materials. Biomaterials 129:1–27. https://doi.org/10.1016/j.biomaterials.2017.03.014
Serizawa T, Fukuta H, Date T, Sawada T (2016) Affinity-based release of polymer-binding peptides from hydrogels with the target segments of peptides. Chem Commun 52(11):2241–2244. https://doi.org/10.1039/c5cc09016d
Burdick JA, Mauck RL (2010) Biomaterials for tissue engineering applications: a review of the past and future trends. Springer Science & Business Media. Available from: https://play.google.com/store/books/details?id=Zj6ubOFXGgUC
Giglio ED, De Giglio E, Cometa S, Ricci MA, Cafagna D, Savino AM, et al (2011) Ciprofloxacin-modified electrosynthesized hydrogel coatings to prevent titanium-implant-associated infections, Acta Biomaterialia7:882–891. Available from: https://doi.org/10.1016/j.actbio.2010.07.030
Marchesan S, Qu Y, Waddington LJ, Easton CD, Glattauer V, Lithgow TJ, et al () Self-assembly of ciprofloxacin and a tripeptide into an antimicrobial nanostructured hydrogel [Internet]. Vol. 34, Biomaterials. 2013. p. 3678–87. https://doi.org/10.1016/j.biomaterials.2013.01.096
Li J, Xing R, Bai S, Yan X (2019) Recent advances of self-assembling peptide-based hydrogels for biomedical applications. Soft Matter 15(8):1704–1715. https://doi.org/10.1039/c8sm02573h
Bamford KB (1999) Chronic gastrointestinal inflammation. FEMS Immunol Med Microbiol 24(2):161–168. https://doi.org/10.1111/j.1574-695X.1999.tb01277.x
Loftus EV Jr (2004) Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 126(6):1504–1517. https://doi.org/10.1053/j.gastro.2004.01.063
Kornbluth A, Sachar DB, and The Practice Parameters Committee of the American College of Gastroenterology (2010) Ulcerative colitis practice guidelines in adults: American College of gastroenterology, practice parameters committee. Official Journal of the American College of Gastroenterology | ACG 105(3):501. Available from: https://journals.lww.com/ajg/Fulltext/2010/03000/Ulcerative_Colitis_Practice_Guidelines_in_Adults_.6.aspx
Ediger JP, Walker JR, Graff L, Lix L, Clara I, Rawsthorne P, et al (2007) Predictors of medication adherence in inflammatory bowel disease. Am J Gastroenterol 102(7):1417–1426. https://doi.org/10.1111/j.1572-0241.2007.01212.x
Zhang S, Ermann J, Succi MD, Zhou A, Hamilton MJ, Cao B, et al (2015) An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci Transl Med 7(300):300ra128. https://doi.org/10.1126/scitranslmed.aaa5657
Araki T, Mitsuyama K, Yamasaki H, Morita M, Tsuruta K, Mori A, et al (2021) Therapeutic potential of a self-assembling peptide hydrogel to treat colonic injuries associated with inflammatory bowel disease. J Crohns Colitis. https://doi.org/10.1093/ecco-jcc/jjab033
Cao X, Duan L, Hou H, Liu Y, Chen S, Zhang S, et al (2020) IGF-1C hydrogel improves the therapeutic effects of MSCs on colitis in mice through PGE2-mediated M2 macrophage polarization. Theranostics 10(17):7697–7709. https://doi.org/10.7150/thno.45434
Hong L, Chen G, Cai Z, Liu H, Zhang C, Wang F, et al (2022) Balancing microthrombosis and inflammation via injectable protein hydrogel for inflammatory bowel disease. Adv Sci 9(20):e2200281. https://doi.org/10.1002/advs.202200281
Singhal P, Vashisht H, Nisar S, Mehra S, Rattan S (2022) Stimulus responsive soy-protein based hydrogels through grafting HEMA for biomedical applications. Ind Crops Prod 178:114621. Available from: https://www.sciencedirect.com/science/article/pii/S0926669022001042
Cheng Z, Qing R, Hao S, Ding Y, Yin H, Zha G, et al (2021) Fabrication of ulcer-adhesive oral keratin hydrogel for gastric ulcer healing in a rat. Regen Biomater 8(2):rbab008. https://doi.org/10.1093/rb/rbab008
Nelson PT, Soma LA, Lavi E (2002) Microglia in diseases of the central nervous system. Ann Med 34(7–8):491–500. https://doi.org/10.1080/078538902321117698
Nanoscaffold smart drug delivery system may help treatment for neurological disorders (2020). Available from: https://www.nanowerk.com/nanotechnology-news2/newsid=56178.php
Fernandez-Serra R, Gallego R, Lozano P, González-Nieto D (2020) Hydrogels for neuroprotection and functional rewiring: a new era for brain engineering. Neural Regeneration Res 15(5):783–9. https://doi.org/10.4103/1673-5374.268891
Xu J, Duan Z, Qi X, Ou Y, Guo X, Zi L, et al (2020) Injectable gelatin hydrogel suppresses inflammation and enhances functional recovery in a mouse model of intracerebral hemorrhage. Front Bioeng Biotechnol 8:785. https://doi.org/10.3389/fbioe.2020.00785
Hekmatimoghaddam S, Iman M, Shahdadi Sardo H, Jebali A (2019) Gelatin hydrogel containing cerium oxide nanoparticles covered by interleukin-17 aptamar as an anti- inflammatory agent for brain inflammation. J Neuroimmunol 326:79–83. https://doi.org/10.1016/j.jneuroim.2018.11.011
He J, Zhang N, Zhu Y, Jin R, Wu F (2021) MSC spheroids-loaded collagen hydrogels simultaneously promote neuronal differentiation and suppress inflammatory reaction through PI3K-Akt signaling pathway. Biomaterials 265:120448. https://doi.org/10.1016/j.biomaterials.2020.120448
Shukla SK, Sharma AK, Gupta V, Yashavarddhan MH (2019) Pharmacological control of inflammation in wound healing. J Tissue Viability 28(4):218–22. https://doi.org/10.1016/j.jtv.2019.09.002
Wu DQ, Zhu J, Han H, Zhang JZ, Wu FF, Qin XH, et al (2018) Synthesis and characterization of arginine-NIPAAm hybrid hydrogel as wound dressing: In vitro and in vivo study. Acta Biomater 65:305–316. https://doi.org/10.1016/j.actbio.2017.08.048
Gupta A, Kowalczuk M, Heaselgrave W, Britland ST, Martin C, Radecka I (2019) The production and application of hydrogels for wound management: a review. Eur Polym J 111:134–151. Available from: https://www.sciencedirect.com/science/article/pii/S0014305718318317
Kang JI, Park KM (2021) Advances in gelatin-based hydrogels for wound management. J Mater Chem B Mater Biol Med 9(6):1503–1520. https://doi.org/10.1039/d0tb02582h
Zhao CC, Zhu L, Wu Z, Yang R, Xu N, Liang L (2020) Resveratrol-loaded peptide-hydrogels inhibit scar formation in wound healing through suppressing inflammation. Regen Biomater 7(1):99–107. https://doi.org/10.1093/rb/rbz041
Sonamuthu J, Cai Y, Liu H, Kasim MSM, Vasanthakumar VR, Pandi B, et al (2020) MMP-9 responsive dipeptide-tempted natural protein hydrogel-based wound dressings for accelerated healing action of infected diabetic wound. Int J Biol Macromol 153:1058–69. https://doi.org/10.1016/j.ijbiomac.2019.10.236
Dong Y, A S, Rodrigues M, Li X, Kwon SH, Kosaric N, et al (2017) Injectable and tunable gelatin hydrogels enhance stem cell retention and improve cutaneous wound healing. Adv Funct Mater 27(24):1606619. https://doi.org/10.1002/adfm.201606619
Cheng L, Cai Z, Ye T, Yu X, Chen Z, Yan Y, et al (2020) Injectable polypeptide‐protein hydrogels for promoting infected wound healing. Adv Funct Mater, p 2001196. https://doi.org/10.1002/adfm.202001196
Cui T, Li X, He S, Xu D, Yin L, Huang X, et al (2020) Instant Self-Assembly Peptide Hydrogel Encapsulation with Fibrous Alginate by Microfluidics for Infected Wound Healing. ACS Biomater Sci Eng 6(9):5001–5011. https://doi.org/10.1021/acsbiomaterials.0c00581
Gueler F, Gwinner W, Schwarz A, Haller H (2004) Long-term effects of acute ischemia and reperfusion injury. Kidney Int66(2):523–527. https://doi.org/10.1111/j.1523-1755.2004.761_11.x
Lutz J, Thürmel K, Heemann U (2010) Anti-inflammatory treatment strategies for ischemia/reperfusion injury in transplantation. J Inflamm7:27. https://doi.org/10.1186/1476-9255-7-27
Chen S, Liu S, Zhang L, Han Q, Liu H, Shen J, et al (2020) Construction of injectable silk fibroin/polydopamine hydrogel for treatment of spinal cord injury. Chem Eng J399:125795. https://www.sciencedirect.com/science/article/pii/S1385894720319239
Lu HD, Charati MB, Kim IL, Burdick JA (2012) Injectable shear-thinning hydrogels engineered with a self-assembling Dock-and-Lock mechanism. Biomaterials33(7):2145–2153. https://doi.org/10.1016/j.biomaterials.2011.11.076
Libby P (2006) Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr83(2):456S–460S. https://doi.org/10.1093/ajcn/83.2.456S
Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med352(16):1685–1695. https://doi.org/10.1056/NEJMra043430
Lawler PR, Bhatt DL, Godoy LC, Lüscher TF, Bonow RO, Verma S, et al (2021) Targeting cardiovascular inflammation: next steps in clinical translation. Eur Heart J42(1):113–131. https://doi.org/10.1093/eurheartj/ehaa099
Peppas NA, Hilt JZ, Khademhosseini A, Langer R (2006) Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv Mater18(11):1345–1360. https://doi.org/10.1002/adma.200501612
Moon JJ, Saik JE, Poché RA, Leslie-Barbick JE, Lee SH, Smith AA, et al (2010) Biomimetic hydrogels with pro-angiogenic properties. Biomaterials 31(14):3840–3847. https://doi.org/10.1016/j.biomaterials.2010.01.104
Seliktar D (2010) Designing cell-compatible hydrogels for biomedical applications. Science 336(6085):1124–1128. https://doi.org/10.1126/science.1214804
Van Vlierberghe S, Dubruel P, Schacht E (2011) Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules12(5):1387–1408. https://doi.org/10.1021/bm200083n
Cai H, Wu FY, Wang QL, Xu P, Mou FF, Shao SJ, et al (2019) Self-assembling peptide modified with QHREDGS as a novel delivery system for mesenchymal stem cell transplantation after myocardial infarction. FASEB J33(7):8306–8320. https://doi.org/10.1096/fj.201801768RR
Reis LA, Chiu LLY, Wu J, Feric N, Laschinger C, Momen A, et al (2015) Hydrogels With Integrin-Binding Angiopoietin-1–Derived Peptide, QHREDGS, for Treatment of Acute Myocardial Infarction. Circ Heart Fail 8(2):333–341. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001881
Firoozi S, Pahlavan S, Ghanian MH, Rabbani S, Tavakol S, Barekat M, et al (2020) A Cell-Free SDKP-Conjugated Self-Assembling Peptide Hydrogel Sufficient for Improvement of Myocardial Infarction. Biomolecules10(2). https://doi.org/10.3390/biom10020205
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al (2018) Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9(6):7204–7218. https://doi.org/10.18632/oncotarget.23208
La Manna S, Di Natale C, Florio D, Marasco D (2018) Peptides as Therapeutic Agents for Inflammatory-Related Diseases. Int J Mol Sci 19(9). https://doi.org/10.3390/ijms19092714
Todoric J, Antonucci L, Karin M (2016) Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev Res9(12):895–905. https://doi.org/10.1158/1940-6207.CAPR-16-0209
Gregory A (2018) Self-assembling peptide hydrogel for local anticancer prodrug delivery in the treatment of glioblastoma multiforme. Clemson University. Available from: https://tigerprints.clemson.edu/all_theses/2941/
Gangrade A, Mandal BB (2019) Injectable carbon nanotube impregnated silk based multifunctional hydrogel for localized targeted and on-demand anticancer drug delivery. ACS Biomater Sci Eng 5(5):2365–2381. https://doi.org/10.1021/acsbiomaterials.9b00416
Yang L, Zhang C, Ren C, Liu J, Zhang Y, Wang J, et al (2019) Supramolecular hydrogel based on chlorambucil and peptide drug for cancer combination therapy. ACS Appl Mater Interfaces 11(1):331–339. https://doi.org/10.1021/acsami.8b18425
Mandal A, Clegg JR, Anselmo AC, Mitragotri S (2020) Hydrogels in the clinic. Bioeng Transl Med 5(2):e10158. https://doi.org/10.1002/btm2.10158
Sharpe LA, Daily AM, Horava SD, Peppas NA (2014) Therapeutic applications of hydrogels in oral drug delivery. Expert Opin Drug Deliv 11(6):901–915. https://doi.org/10.1517/17425247.2014.902047
Madl CM, Heilshorn SC (2017) Tyrosine-selective functionalization for bio-orthogonal cross-linking of engineered protein hydrogels. Bioconjug Chem 28(3):724–730. https://doi.org/10.1021/acs.bioconjchem.6b00720
Vijayavenkataraman S, Yan WC, Lu WF, Wang CH, Fuh JYH (2018) 3D bioprinting of tissues and organs for regenerative medicine. Adv Drug Deliv Rev 132:296–332. https://doi.org/10.1016/j.addr.2018.07.004
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conceptualization of the article. The literature search and the final draft of the manuscript were carried out by JB. The outline draft of the review was prepared by SR.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bertilla, X.J., Rupachandra, S. Insights into current directions of protein and peptide-based hydrogel drug delivery systems for inflammation. Polym. Bull. 80, 9409–9436 (2023). https://doi.org/10.1007/s00289-022-04527-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04527-1