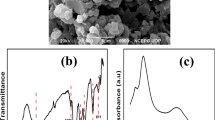
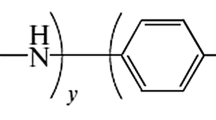
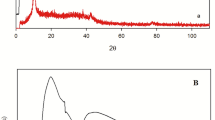
Abstract
Nowadays due to growth in industrialization, hazardous coloured effluents are released in water which affects flora and fauna as well as human life. Due to the scarcity of fresh water on the earth, a lot of scientific approaches were being applied for investigating and removal of these coloured effluents from wastewater. Amongst them, electrochemical sensing and adsorption of such dyes using conducting polymers are the emerging field nowadays. In this regard, a conducting polymer i.e. polyaniline and its composite with graphene oxide were synthesized via an in-situ chemical oxidative method. The electrochemical sensing reveals that polymer composite has high sensitivity towards Congo red as compared to prestige polyaniline. Maximum drop in peak current was noticed for polymer composite i.e. 1.43 mA from 1.98 mA i.e. attributed to a porous surface and maximum active surface area i.e. 3.46E−3 cm2. The adsorption and sensing of Congo red on polyaniline-graphene oxide follow pseudo-second-order kinetics. The charge transfer resistance for composite first increases and then decreases with an increase in dye dosage i.e. due to the redox property of Congo red. The interaction between dye and polymer surface is physical. From the concentration-effect, it has been concluded that adsorption of CR on polyaniline-graphene oxide composite follows the Freundlich isotherm model which indicates the physio-sorption of Congo red i.e. also confirmed by the \(\Delta H\) in the range of 5–80 kJ/mol.










Similar content being viewed by others
References
Arnon TA, Ezra S, Fishbain B (2019) Water characterization and early contamination detection in highly varying stochastic background water, based on machine learning methodology for processing real-time UV-Spectrophotometry. Water Res 155:333–342
Mohamed NA, Al-Harby NF, Almarshed MS (2020) Enhancement of adsorption of Congo red dye onto novel antimicrobial trimellitic anhydride isothiocyanate-cross-linked chitosan hydrogels. Polym Bull 77(12):6135–6160
Brillas E, Martínez-Huitle CA (2015) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl Catal B Environ 166:603–643
Salem MA (2010) The role of polyaniline salts in the removal of direct blue 78 from aqueous solution: a kinetic study. React Funct Polym 70(10):707–714
Padhi B (2012) Pollution due to synthetic dyes toxicity & carcinogenicity studies and remediation. Int J Environ Sci 3(3):940–955
Munagapati VS, Kim D-S (2016) Adsorption of anionic azo dye Congo Red from aqueous solution by Cationic Modified Orange Peel Powder. J Mol Liq 220:540–548
Jiang C, Fu B, Cai H, Cai T (2016) Efficient adsorptive removal of Congo red from aqueous solution by synthesized zeolitic imidazolate framework-8. Chem Speciat Bioavailab 28(1–4):199–208
Mohan SV, Ramanaiah S, Sarma P (2008) Biosorption of direct azo dye from aqueous phase onto Spirogyra sp. I02: evaluation of kinetics and mechanistic aspects. Biochem Eng 38(1):61–69
Zhu T, Song Y, Ji H, Xu Y, Song Y, Xia J, Yin S, Li Y, Xu H, Zhang Q (2015) Synthesis of g-C3N4/Ag3VO4 composites with enhanced photocatalytic activity under visible light irradiation. Chem Eng J 271:96–105
An H, Li M, Gao J, Zhang Z, Ma S, Chen Y (2019) Incorporation of biomolecules in metal-organic frameworks for advanced applications. Coord Chem Rev 384:90–106
Mólgora CC, Domínguez AM, Avila EM, Drogui P, Buelna G (2013) Removal of arsenic from drinking water: a comparative study between electrocoagulation-microfiltration and chemical coagulation-microfiltration processes. Sep Purif Technol 118:645–651
Lim SJ, Kim T-H (2015) Combined treatment of swine wastewater by electron beam irradiation and ion-exchange biological reactor system. Sep Purif Technol 146:42–49
Mirmohseni A, Oladegaragoze A (2000) Anti-corrosive properties of polyaniline coating on iron. Synth Met 114(2):105–108
Mahanta D, Munichandraiah N, Radhakrishnan S, Madras G, Patil S (2011) Polyaniline modified electrodes for detection of dyes. Synth Met 161(9–10):659–664
Ma Y, Guo J, Chen Y, Yi Y, Zhu G (2021) Electrochemical sensing of phenolics based on copper/cobalt/nitrogen co-doped hollow nanocarbon spheres. J Electroanal Chem 892:115263
Tovide OO (2013) Graphenated polyaniline nanocomposite for the determination of polyaromatic hydrocarbons (PAHs) in water
Shahrokhian S, Zare-Mehrjardi HR (2009) Electrochemical synthesis of polypyrrole in the presence of congo red; application to selective voltammetric determination of dopamine in the presence of ascorbic acid. Electroanalysis 21(2):157–164. https://doi.org/10.1002/elan.200804424
Sun J, Gan T, Li Y, Shi Z, Liu Y (2014) Rapid and sensitive strategy for Rhodamine B detection using a novel electrochemical platform based on core–shell structured Cu@ carbon sphere nanohybrid. J Electroanal Chem 724:87–94
Rehman TU, Shah LA (2019) Rheological investigation of GO doped p(APTMACl) composite hydrogel. Z Phys Chem 235(3):329–343. https://doi.org/10.1515/zpch-2019-1416
Karaoğlan N, Bindal C (2018) Synthesis and optical characterization of benzene sulfonic acid doped polyaniline. Eng Sci Technol Int J 21(6):1152–1158
Vats S, Kumar A, Kalia S, Mohammad J, Prasad N (2022) Synthesis of bismuth doped cobalt ferrite and its composite with polyaniline. Mater Today Proc 48:679–682
Wei H, Zhu J, Wu S, Wei S, Guo Z (2013) Electrochromic polyaniline/graphite oxide nanocomposites with endured electrochemical energy storage. Polymer 54(7):1820–1831
Khan M, Shah LA, Khan MA, Khattak NS, Zhao H (2020) Synthesis of an un-modified gum arabic and acrylic acid based physically cross-linked hydrogels with high mechanical, self-sustainable and self-healable performance. Mater Sci Eng C 116:111278
Jain R, Sinha A, Kumari N (2016) A polyaniline/graphene oxide nanocomposite as a voltammetric sensor for electroanalytical detection of clonazepam. Anal Methods 8(15):3034–3045
Shetti NP, Malode SJ, Malladi RS, Nargund SL, Shukla SS, Aminabhavi TM (2019) Electrochemical detection and degradation of textile dye Congo red at graphene oxide modified electrode. Microchem 146:387–392
Radhakrishnan S, Krishnamoorthy K, Sekar C, Wilson J, Kim SJ (2015) A promising electrochemical sensing platform based on ternary composite of polyaniline–Fe2O3–reduced graphene oxide for sensitive hydroquinone determination. Chem Eng J 259:594–602
Gök ZG, Günay K, Arslan M, Yiğitoğlu M (2019) Removing of Congo red from aqueous solution by 2-hydroxyethyl methacrylate-g-poly (ethylene terephthalate) fibers. Polym Bull 76(12):6179–6191
Faizan S, Shah LA (2021) Facile fabrication of hydrogels for removal of crystal violet from wastewater. Int J Environ Sci Technol 19(6):4815–4826. https://doi.org/10.1007/s13762-021-03454-4
Shu Y, Wang J, Qian C, Shi Q, Lv R, Wu H, Chen M (2021) Dibenzo-18-crown-6/Polyacrylonitrile (PAN) nanofibers for metal ions adsorption: adsorption studies for Na+ and K+. Polym Bull 79:1–14
Rehman TU, Shah LA, Khan M, Irfan M, Khattak NS (2019) Zwitterionic superabsorbent polymer hydrogels for efficient and selective removal of organic dyes. RSC Adv 9(32):18565–18577
Subhan H, Alam S, Shah LA, Ali MW, Farooq M (2021) Sodium alginate grafted poly (N-vinyl formamide-co-acrylic acid)-bentonite clay hybrid hydrogel for sorptive removal of methylene green from wastewater. Colloids Surf A Physicochem Eng Asp 611:125853
Acknowledgements
Institute of Chemical Science, University of Peshawar and National Centre of Excellence in Physical Chemistry, University of Peshawar are gratefully acknowledged for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest exists to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Faizan, S., Bakhtawara & Zaid, F. Detection and removal of Congo red via aniline-based polymer and polymer composite. Polym. Bull. 80, 7971–7989 (2023). https://doi.org/10.1007/s00289-022-04432-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04432-7