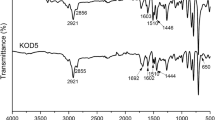
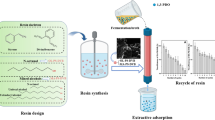
Abstract
Phenols are commonly encountered in the aqueous effluents of different industrial processes, such as oil refining, and phenolic resin manufacturing and processing. Because phenols are toxic, they should be promptly removed from aquatic environments. Herein, we describe the preparation of photocrosslinked β-cyclodextrin (β-CyD) polymer beads and their use for the removal of phenol (PhOH) from the raw industrial wastewater from phenolic resin processing. Polysubstituted photocrosslinkable β-CyD (PSβCyD/UV) sorbent beads were obtained via the reaction of globed macromonomers with sodium alginate and calcium chloride followed by photocrosslinking the β-cyclodextrin (β-CyD) macromonomers with isophorone diisocyanate and 2-hydroxyethyl acrylate (molar ratio of 1:3.5:3.5). The physical properties of the fabricated PSβCyD/UV beads were as follows: average diameter, 2.7 mm; average compressive strength, 6.5 M Pa; porosity, 41.4%; and specific surface area, 2.89 m2/g. The removal of PhOH from raw industrial wastewater using β-CyD polymer beads was performed in a shaker at 25 °C. After six accumulated adsorption cycles, the PhOH concentration decreased from of 89,000 to 1500 mg/L; furthermore, the expansion factor of PSβCyD/UV was 1.33. Adsorbent PSβCyD/UV polymer beads with a regular spherical shape and high mechanical stability were prepared using a photocrosslinking method. The results of the sorption experiments indicated that the PhOH adsorption capacity of the polymer beads from raw industrial wastewater was high (2.43 mmol/g-resin). We concluded that the prepared polymer beads formed rich polymer network structures, which affected the physical adsorption of PhOH and the chemical interactions with PhOH via hydrogen bonding.







Similar content being viewed by others
References
Himmelstein KJ, Fox RD, Winter TH (1973) In-place regeneration of activated carbon. Chem Eng Prog 69:65–69
Hashimoto K, Miura K (1975) Intraparticle diffusivities in liquid-phase adsorption with nonlinear isotherms. J Chem Eng Jap 8:367–373. https://doi.org/10.1252/jcej.8.36
Yamasaki H, Fukunaga K, Kawatsu T, Miyoshi R (2005) Method for removing phenolic components. Japanese Published Patent, No, p 2005125193
Szejtli J (1998) Introduction and general overview of cyclodextrin chemistry. Chem Rev 98:1743–1753. https://doi.org/10.1021/cr970022c
Crini G, Morcellet M (2002) Synthesis and applications of adsorbents containing cyclodextrins. J Sep Sci 25:789–813. https://doi.org/10.1002/1615-9314(20020901)25:13%3c789::AID-JSSC789%3e3.0.CO;2-J
Cichy W, Szymanowski J (2002) Recovery of phenol from aqueous streams in hollow fiber modules. J Environ Sci Technol 36:2088–2093. https://doi.org/10.1021/es010910w
Kiji J, Konishi H, Okano T, Terashima T, Motomura K (1992) Adsorption of organic species by a cyclodextrin epichlorohydrin network polymer. Die Angew Makromo Chemie 199:207–210. https://doi.org/10.1002/apmc.1992.051990116
Li D, Ma M (1999) Nanosponges: From inclusion chemistry to water purifying technology. CHEMTECH 2931-37
Lee KP, Choi SH, Ryu E-N, Ryoo JJ, Park JH, Kim Y, Hyun MH (2002) Preparation and characterization of cyclodextrin polymer and its high-performance liquid-chromatography stationary phase. Anal Sci 18:31–34
Crini G, Bertini S, Torri G, Naggi A, Sforzini D, Vecchi C, Janus L, Lekchiri Y, Morcellet M (1998) Sorption of aromatic compounds in water using insoluble cyclodextrin polymers. J Appl Polym Sci 68:1973–1978. https://doi.org/10.1002/(SICI)1097-4628(19980620)68:12%3c1973::AID-APP11%3e3.0.CO;2-T
Friedman RB, Hedges AR, Black FL, Gottneid DJ (1989) Complexation of aromatic compounds with, and their release from, cyclomaltoheptaose-containing polymers, hydroxyethylcyclomaltoheptaose, and cyclomaltoheptaose. Carbohydr Res 192:283–289
Carbonnier B, Janus L, Lekchiri Y, Morcellet M (2004) Coating of porous silica beads by in situ polymerization/crosslinking of 2-hydroxypropyl β-cyclodextrin for reversed-phase high performance liquid chromatography applications. J Appl Polym Sci 91:1419–1426. https://doi.org/10.1002/app.13306
Nishiki M, Tojima T, Nishi N, Sakairi N (2000) β-Cyclodextrin-linked chitosan beads: preparation and application to removal of bisphenol A from water. Carbohydr Lett 4:61–67. https://pubmed.ncbi.nlm.nih.gov/11469339/
Yamasaki H, Makihata Y, Fukunaga K (2006) Efficient phenol removal of wastewater from phenolic resin plants using crosslinked cyclodextrin particles. J Chem Technol Biotechnol 81:1271–1276. https://doi.org/10.1002/jctb.1545
Yamasaki H, Odamura A, Makihata Y, Fukunaga K (2017) Preparation of new photo-crosslinked β-cyclodextrin polymer beads. Polym J 49:377–383. https://doi.org/10.1038/pj.2016.127
Yamasaki H, Matsui H, Fukunaga K (2007) Efficient phenol removal of raw industrial wastewater from phenolic resin plants using crosslinked β-cyclodextrin adsorbent. Japanese Environ Conser Eng 36:50–56. https://doi.org/10.5956/jriet.36.282
Yamasaki H, Makihata Y, Fukunaga K (2008) Preparation of crosslinked β-cyclodextrin polymer beads and their application as a sorbent for removal of phenol from wastewater. J Chem Technol Biotechnol 83:991–997. https://doi.org/10.1002/jctb.1904
Yamasaki H, Fukunaga K (2012) Development of the environmental symbiosis material utilizing the property of cyclodextrin. Japanese Environ Conser Eng 41:679–682
Yamasaki H, Fukunaga K (2021) Phenol recovery from industrial wastewater by β-CyD polymer beads. Japanese Environ Conser Eng 50:93–98. https://doi.org/10.5956/jriet.50.2_93
Brunauer S, Emmett P, Teller E (1938) B E T method. J Am Chem Soc 60:309
Yadav LS, Mishra BK, Arvind K (2019) Adsorption of phenol from aqueous solutions by bael furit shell activated carbon: Kinetic, equilibrium, and mass transfer studies. Theor Found Chem Eng 53:122–131. https://doi.org/10.1134/S0040579519010184
Ocampo-Pe´rez R, Leyva-Ramos R, Sanchez-Polo M, Rivera-Utrilla J (2013) Role of pore volume and surface diffusion in the adsorption of aromatic compounds on activated carbon. Adsorption 19:945–957
Crini G, Janus L, Morcellet M, Torri G, Morin N (1999) Sorption properties toward substituted phenolic derivative in water using macroporous polyamines containing β-cyclodextrin. J Appl Polym Sci 73:2903–2910. https://doi.org/10.1002/(SICI)1097-4628(19990929)73:14%3c2903::AID-APP14%3e3.0.CO;2-2
Abay I, Denizli A, Bişkin E, Salih B (2005) Removal and pre-concentration of phenolic species onto β-cyclodextrin modified poly(hydroxyethylmethacrylate–ethyleneglycoldimethacrylate) microbeads. Chemosphere 61:1263–1272. https://doi.org/10.1016/j.chemosphere.2005.03.079
Acknowledgments
This work was partly supported by a Grant-in-Aid for Young Scientists (B) (No. 15710069) from the Ministry of Education, Culture, Sports, Science and Technology Japan (2003–2005), and by a research promotion grant from the Conference for Reduction of Energy & Heat-trapping Gas in the Ube Industrial Complex (2005–2008). The authors are grateful to Mercian Co., Ltd. (Tokyo) and Meiwa Plastic Industries, Ltd. (Ube) for the provision of RINGDEX-B and the phenolic wastewater, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamasaki, H., Odamura, A., Makihata, Y. et al. Photocrosslinked β-cyclodextrin polymer beads and their use as sorbent for phenol removal from wastewater. Polym. Bull. 80, 3265–3278 (2023). https://doi.org/10.1007/s00289-022-04206-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04206-1