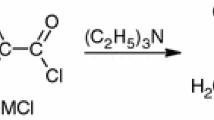
Abstract
Vinyl sulfonic acid (VSA) monomer at various pH values was polymerized by using a special radical initiator 2,2′-azobis(2-methylpropanediamine) dihydrochloride in 50 °C and 50 h by free radical polymerization method. The gravimetric conversions were obtained, and it was found that the conversion was the maximum in acidic media and the minimum in basic media. The structural behavior of poly(vinyl sulfonic acid) P(VSA) and poly(vinyl sulfonic acid sodium salt) P(VSANa) polymers were investigated by Fourier Transform Infrared (FT-IR), Raman, Nuclear Magnetic Resonance (NMR) (1H, 13C, Distortionless Enhancement by Polarization Transfer (135DEPT) and Homo Nuclear Correlation spectroscopy, (Cosy) and thermal properties were obtained by Thermal Gravimetry Analysis (TGA). It was found molecular weight approximately 6000 g/mol by 1H-NMR spectroscopy method for P(VSANa). Furthermore, for investigation of the microstructure of P(VSA) acid and P(VSANa), we used the Matrix-assisted Laser Desorption Ionization Time of Flight (MALDI-TOF) spectroscopy method and Cosy NMR spectroscopy. The Microstructure for P(VSA) was found in cyclic form. The molecular weights for two homopolymers were obtained at 2300 g/m by MALDI-TOF spectroscopy.















Similar content being viewed by others
References
Borup R, Meyers J, Pivovar B, Kim YS, Mukundan R, Garland N, Myers D, Wilson M, Garzon F, Wood D, Zelenay P, More K, Stroh K, Zawadzinski T, Boncella J, McGrath JE, İnaba M, Miyatake K, Hori M, Ota K, Ogumi Z, Miyata S, Nishikata A, Siroma Z, Uchimoto Y, Yasuda K, Kimijima K, Iwashita N (2007) Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem Rev 107(10):3904–3951. https://doi.org/10.1021/cr050182l
Rivas BL, Pereira ED, Gallegos P, Geckeler KE (2002) Water-soluble acidic polyelectrolytes with metal–removing ability. Polym Adv Technol 13:1000–1005. https://doi.org/10.1002/pat.247
Rivas BL, Seguel GV, Geckeler KE (2002) Synthesis characterization, and properties of polychelates of poly(styrene sulfonic acid-co-maleic acid) with Co+2, Cu+2, Ni+2. J Appl Polym Sci 85:2546–2551. https://doi.org/10.1002/app.10862
Saito T, Moore HD, Hickner MA (2010) Synthesis of midblock-sulfonated Triblock copolymers. Macromolecules 43(2):599–601. https://doi.org/10.1021/ma9023125
Kusunoki T, Oshiro M, Hamasaki T, Kobayashi T (2011) Polyvinylphosphonic acid copolymer hydrogels prepared with amide and ester type crosslinkers. J Appl Polym Sci 119(5):3072–3079. https://doi.org/10.1002/app.33053
Yameen B, Kaltbeitzel A, Langer A, Muller F, Gosele U, Knoll W, Azzaroni O (2009) Hily proton- conducting self-humidifying microchannels generated by copolymer brushes an scffold. Angew Chem Int Ed 48:3124–3128. https://doi.org/10.1002/anie.200805576
Tago T, Shibata H, Nishide H (2007) Proton conductivity in the dry membrane of poly(sulfonic acid) and polyamine layer-by-layer complex. Chem Commun 28:2989–2991. https://doi.org/10.1039/B703947F
Friedel B, Keivanidis PE, Brenner TJK, Abrusci A, McNeill CR, Friend RH, Greenham NC (2009) Effect of layer thickness and annealing of PEDOT:PSS layers in organic photodetectors. Macromolecues 42(17):6741–6747. https://doi.org/10.1021/ma901182u
Okuzaki H, Suzuki H, Ito K (2009) Electromechanical properties of poly(3,4 ethylendioxthiophene)/poly(4 styrene sulfonate) films. J Phys Chem B 113(33):11378–11383. https://doi.org/10.1021/jp902845x
Lange U, Mirsky VM (2011) Chemosensitive nanocomposite for conductometric detection of hydrazine and NADH. Electrochim Acta 56(10):3679–3684. https://doi.org/10.1016/j.electacta.2010.08.092
Argentiere S, Blasi L, Ciccarella G, Barbarella G, Cingolani R, Gigli G (2010) Nanogels of poly(acrylic acid): Uptake and release behavior with fluorescent oligothiophene-labeled bovine serum albumin. J Appl Polym Sci 116(5):2808–2815. https://doi.org/10.1002/app.31691
Okayasu T, Hibino T, Nishide H (2011) Free radical polymerization kinetics of vinylsulfonic acid and highly acidic properties of its polymer. Macromol Chem Phys 212(10):1072–1079. https://doi.org/10.1002/macp.201000773
Nilles K, Theato P (2011) Polymerization of an activated ester monomer based on 4-vinylsulfonic acid and its polymer analogous reaction. Polym Chem 2(2):376–384. https://doi.org/10.1039/C0PY00261E
Jung JH, Jeon JH, Sridhar V, Oh IK (2011) Electro-active graphene–Nafion actuators. Carbon 49(4):1279–1289. https://doi.org/10.1016/j.carbon.2010.11.047
Chai Z, Wang C, Zhang H, Doherty CM, Ladewig BP, Hill AJ, Wang H (2010) Nafion–carbon nanocomposite membranes prepared using hydrothermal carbonization for proton-exchange-membrane fuel cells. Adv Funct Mater 20(24):4394–4399. https://doi.org/10.1002/adfm.201001412
Dong B, Gwee L, de la Cruz DS, Winey KI, Elabd YA (2010) Super proton conductive high-purity Nafion nanofibers. Nano Lett 10(9):3785–3790. https://doi.org/10.1021/nl102581w
Azad UP, Ganesan V (2010) Efficient sensing of nitrite by Fe (bpy) 32+ immobilized Nafion modified electrodes. Chem Commun 46(33):6156–6158. https://doi.org/10.1039/C0CC00852D
Panawong C, Martwiset S (2018) Synthesis and characterization of poly(styrene sulfonic acid-co-1-vinylimidazole-co-styrene) and its blends with poly(vinyl chloride) as proton conducting membranes. Polym Bull 75:3843–3858. https://doi.org/10.1007/s00289-017-2240-7
Choudhury RR, Gohil JM, Dutta K (2021) Poly(vinyl alcohol)-based membranes for fuel cell and water treatment applications: a review on recent advancements. Polym Adv Technol 32(11):4175–4203. https://doi.org/10.1002/pat.5431
Okayasu T, Saito K, Nishide H, Hearn MTW (2009) Preparation of a novel poly (vinylsulfonic acid)-grafted solid phase acid catalyst and its use in esterification reactions. Chem Comm 31:4708–4710. https://doi.org/10.1039/b823177j
Kim SJ, Park SJ, Kim SI (2004) Properties of smart hydrogels composed of polyacrylic acid/poly (vinyl sulfonic acid) responsive to external stimuli. Smart Mater Struct 13(2):317–322. https://doi.org/10.1088/0964-1726/13/2/010
Pozdnyakov AS, Sekretarev EA, Emel’yanov AI, Prozorova GF (2017) Hydrophilic functional copolymers of 1-vinyl-1,2,4-triazole with vinylsulfonic acid sodium salt. Russ Chem 66(12):2293–2297. https://doi.org/10.1007/s11172-017-2017-z
Yadav M, Srivastav A, Verma SK, Behari K (2013) Graft (partially carboxymethylated guar gum-g-poly vinyl sulfonic acid) copolymer: From synthesis to applications. Carbohydr Polym 97(2):597–603. https://doi.org/10.1016/j.carbpol.2013.02.084
Sand A, Yadav M, Behari K (2010) Synthesis and characterization of alginate-g-vinyl sulfonic acid with a potassium peroxydiphosphate/thiourea system. J Appl Polym Sci 118:3685–3694. https://doi.org/10.1002/app.32447
Van Vernon A, Edward HW (1940). Ethylenesulphonyl polymer, US Patent, 2348705A, filled April 30, 1940, issued May 16, 1944.
Bingöl B, Meyer WH, Wagner M, Wegner G (2006) Synthesis, microstructure, and acidity of poly(vinylphosphonic acid). Macromol Rapid Comm 27(20):1719–1724. https://doi.org/10.1002/Marc.200600513
Overberger CG, Baldwin DE, Gregor HP (1950) Copolymerization of butyl vinylsulfonate. Comments on sulfur shell expansion1. J Am Chem Soc 72(11):4864–4866. https://doi.org/10.1021/ja01167a005
Breslow DS, Hough RR, Fairclough JT (1954) Synthesis of sodium ethylenesulfonate from ethanol. J Am Chem Soc 76(21):5361–5363. https://doi.org/10.1021/ja01650a033
Breslow DS, Kutner A (1958) Polymers and copolymers of sodium ethylenesulfonate. J Polym Sci 27(115):295–312. https://doi.org/10.1002/pol.1958.1202711525
Kern W, Schulz RC (1957) Synthetische makromolekulare Stoffe mit reaktiven Gruppen. Angew Chem Int 69(5):153–171. https://doi.org/10.1002/ange.19570690502
Skoog DA, Holler FJ, Crouch SR (2017) Principles of instrumental analysis. Cengage learning. Jan 27
Tanaka N, Kitano H, Ise N (1989) Raman spectroscopic study of the ionization of polyelectrolytes. 1. Poly (styrenesulfonic acid) and poly(ethylenesulfonic acid). Macromolecules 22(6):2652–2656. https://doi.org/10.1021/ma00196a020
Marvel CS, Menikheim VC, Inskip HK, Taft WK, Labbe BG (1953) Copolymerization of vinylsulfonic acid derivatives with butadiene. J Polym Sci Part B 10(1):39–48. https://doi.org/10.1002/pol.1953.120100104
Sepulveda VR, Sierra L, López BL (2018) Low Dispersity and high conductivity poly (4-styrenesulfonic acid) membranes obtained by inexpensive free radical polymerization of sodium 4-styrenesulfonate. Membranes 8(3):58. https://doi.org/10.3390/membranes8030058
Jiang DD, Yao Q, McKinney MA, Wilkie C (1999) TGA/FTIR studies on the thermal degradation of some polymeric sulfonic and phosphonic acids and their sodium salts. Polym Degrad Stab 63(3):423–434. https://doi.org/10.1016/S0141-3910(98)00123-2
Nguyen TH, Paluck SJ, McGahran AJ, Maynard HD (2015) Poly (vinyl sulfonate) facilitates bFGF-induced cell proliferation. Biomacromol 16(9):2684–2692. https://doi.org/10.1021/acs.biomac.5b00557
Izunobi JU, Higginbotham CL (2011) Polymer molecular weight analysis by 1HNMR spectroscopy. J Chem Educ 88(8):1098–1104. https://doi.org/10.1021/ed100461v
Öztop HN, Akyildiz F, Saraydin D (2020) Poly (acrylamide/vinylsulfonic acid) hydrogel for invertase immobilization. Microsc Res Tech 83(12):1487–1498. https://doi.org/10.1002/jemt.23542
Rabek JF (1990) Experimental methods in polymer chemistry-physical principles and applications. John Wiley, New york, pp 189–194
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sepehrianazar, A., Güven, O. Synthesis and characterization of poly(vinyl sulfonic acid) in different pH values. Polym. Bull. 80, 3005–3020 (2023). https://doi.org/10.1007/s00289-022-04190-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04190-6