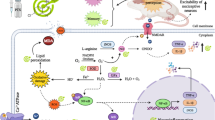
Abstract
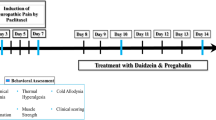
Although chemotherapy-induced peripheral neuropathy is one of the main medical complaints of cancer patients, there are currently no specific drugs for the effective treatment. In this respect, α-terpineol (TP) seems to be a potential candidate for this purpose, since it has several pharmacological properties that can be improved by nanosytems. Therefore, the objective of this study was to develop and investigate an antinociceptive action of TP in nanocapsules (TP-LNC) on a peripheral neuropathy induced by paclitaxel (PTX) in mice. Nanocapsules were obtained through the interfacial deposition of preformed polymer method and physicochemically characterized. The effect of TP and TP-LNC was assessed using the hyperalgesia test in mice with neuropathy induced by PTX (32 mg/kg, i.p.). Its effect on calcium channels was evaluated by patch-clamp and molecular docking. TP-LNC presented satisfactory stability and encapsulation efficiency of 64.5%; it also prolonged the antihyperalgesic effect when cooperated with free TP. Moreover, it was found that TP presented molecular interactions with different channels for calcium, being able to reduce calcium currents. These findings bring new evidence about the analgesic action of TP and its modulating action of calcium channels and reiterate the benefits of the encapsulation of monoterpenes in nanosystems.




Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Jemal A, Torre L, Soerjomataram I, Bray F (2019) The Cancer Atlas, 3rd edn. American Cancer Society, Atlanta. ISBN 978-1-60443-265-7
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL (2019) Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 69:363–385. https://doi.org/10.3322/caac.21565
Opzoomer JW, Sosnowska D, Anstee JE, Spicer JF, Arnold JN (2019) Cytotoxic chemotherapy as an immune stimulus: a molecular perspective on turning up the immunological heat on cancer. Front Immunol. https://doi.org/10.3389/fimmu.2019.01654
Nurgali K, Jagoe RT, Abalo R (2018) Editorial: adverse effects of cancer chemotherapy: anything new to improve tolerance and reduce sequelae? Front Pharmacol. https://doi.org/10.3389/fphar.2018.00245
Cavaletti G, Alberti P, Marmiroli P (2015) Chemotherapy-induced peripheral neurotoxicity in cancer survivors: an underdiagnosed clinical entity? Am Soc Clin Oncol Educ Book Am Soc Clin Oncol Annu Meet. https://doi.org/10.14694/EdBook_AM.2015.35.e553
Cella D, Peterman A, Hudgens S, Webster K, Socinski MA (2003) Measuring the side effects of taxane therapy in oncology: the functional assessment of cancer therapy-taxane (FACT-taxane). Cancer 98:822–831. https://doi.org/10.1002/cncr.11578
Sałat K (2020) Chemotherapy-induced peripheral neuropathy: part 1—current state of knowledge and perspectives for pharmacotherapy. Pharmacol Rep 72:486–507. https://doi.org/10.1007/s43440-020-00109-y
Miaskowski C, Mastick J, Paul SM, Abrams G, Cheung S, Sabes JH, Kober KM, Schumacher M, Conley YP, Topp K et al (2018) Impact of chemotherapy-induced neurotoxicities on adult cancer survivors’ symptom burden and quality of life. J Cancer Surviv Res Pract 12:234–245. https://doi.org/10.1007/s11764-017-0662-8
Portenoy RK, Ahmed E (2018) Cancer pain syndromes. Hematol Oncol Clin North Am 32:371–386. https://doi.org/10.1016/j.hoc.2018.01.002
Scripture CD, Figg WD, Sparreboom A (2006) Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Curr Neuropharmacol 4:165–172
Gouveia DN, Pina LTS, Rabelo TK, da Rocha Santos WB, Quintans JSS, Guimaraes AG (2018) Monoterpenes as perspective to chronic pain management: a systematic review. Curr Drug Targets 19:960–972. https://doi.org/10.2174/1389450118666170711145308
Gouveia DN, Guimarães AG, da Santos WBR, Quintans-Júnior LJ (2019) Natural products as a perspective for cancer pain management: a systematic review. Phytomedicine Int J Phytother Phytopharm 58:152766. https://doi.org/10.1016/j.phymed.2018.11.026
Santos WBR, Melo MAO, Alves RS, de Brito RG, Rabelo TK, da Prado LS, Silva VKDS, Bezerra DP, de Menezes-Filho JER, Souza DS et al (2019) P-cymene attenuates cancer pain via inhibitory pathways and modulation of calcium currents. Phytomedicine Int J Phytother Phytopharm. 61:152836. https://doi.org/10.1016/j.phymed.2019.152836
Brami C, Bao T, Deng G (2016) Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: a systematic review. Crit Rev Oncol Hematol 98:325–334. https://doi.org/10.1016/j.critrevonc.2015.11.014
Khaleel C, Tabanca N, Buchbauer G (2018) α-Terpineol, a natural monoterpene: a review of its biological properties. Open Chem 16:349–361. https://doi.org/10.1515/chem-2018-0040
de Oliveira MGB, Marques RB, de Santana MF, Santos ABD, Brito FA, Barreto EO, De Sousa DP, Almeida FRC, Badauê-Passos D, Antoniolli AR et al (2012) α-terpineol reduces mechanical hypernociception and inflammatory response. Basic Clin Pharmacol Toxicol 111:120–125. https://doi.org/10.1111/j.1742-7843.2012.00875.x
Quintans-Júnior LJ, Oliveira MGB, Santana MF, Santana MT, Guimarães AG, Siqueira JS, De Sousa DP, Almeida RN (2011) α-terpineol reduces nociceptive behavior in mice. Pharm Biol 49:583–586. https://doi.org/10.3109/13880209.2010.529616
Oliveira MGB, Brito RG, Santos PL, Araújo-Filho HG, Quintans JSS, Menezes PP, Serafini MR, Carvalho YMBG, Silva JC, Almeida JRGS et al (2016) α-terpineol, a monoterpene alcohol, complexed with β-cyclodextrin exerts antihyperalgesic effect in animal model for fibromyalgia aided with docking study. Chem Biol Interact 254:54–62. https://doi.org/10.1016/j.cbi.2016.05.029
Gouveia DN, Costa JS, Oliveira MA, Rabelo TK, de Oliveira e Silva AM, Carvalho AA, Miguel-Dos-Santos R, Lauton-Santos S, Scotti L, Scotti MT et al (2018) α-terpineol reduces cancer pain via modulation of oxidative stress and inhibition of INOS. Biomed Pharmacother Biomedecine Pharmacother. 105:652–661. https://doi.org/10.1016/j.biopha.2018.06.027
Soleimani M, Sheikholeslami MA, Ghafghazi S, Pouriran R, Parvardeh S (2019) Analgesic effect of α-terpineol on neuropathic pain induced by chronic constriction injury in rat sciatic nerve: involvement of spinal microglial cells and inflammatory cytokines. Iran J Basic Med Sci 22:1445–1451. https://doi.org/10.22038/IJBMS.2019.14028
Bilia AR, Guccione C, Isacchi B, Righeschi C, Firenzuoli F, Bergonzi MC (2014) Essential oils loaded in nanosystems: a developing strategy for a successful therapeutic approach. Evid-Based Complement Altern Med ECAM 2014:651593. https://doi.org/10.1155/2014/651593
Kothamasu P, Kanumur H, Ravur N, Maddu C, Parasuramrajam R, Thangavel S (2012) Nanocapsules: the weapons for novel drug delivery systems. Bioimpacts 2:71–81. https://doi.org/10.5681/bi.2012.011
Prasad M, Lambe UP, Brar B, Shah I, Manimegalai J, Ranjan K, Rao R, Kumar S, Mahant S, Khurana SK et al (2018) Nanotherapeutics: an insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed Pharmacother 97:1521–1537. https://doi.org/10.1016/j.biopha.2017.11.026
Beiranvand S, Sorori MM (2019) Pain management using nanotechnology approaches. Artif Cells Nanomedicine Biotechnol 47:462–468. https://doi.org/10.1080/21691401.2018.1553885
Chen J, Jin T, Zhang H (2020) Nanotechnology in chronic pain relief. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2020.00682
Clemente-Napimoga JT, Moreira JA, Grillo R, de Melo NFS, Fraceto LF, Napimoga MH (2012) 15d-PGJ2-loaded in nanocapsules enhance the antinociceptive properties into rat temporomandibular hypernociception. Life Sci 90:944–949. https://doi.org/10.1016/j.lfs.2012.04.035
Ediriwickrema A, Saltzman WM (2015) Nanotherapy for cancer: targeting and multifunctionality in the future of cancer therapies. ACS Biomater Sci Eng 1:64–78. https://doi.org/10.1021/ab500084g
Villalba BT, Ianiski FR, Wilhelm EA, Fernandes RS, Alves MP, Luchese C (2014) Meloxicam-loaded nanocapsules have antinociceptive and antiedematogenic effects in acute models of nociception. Life Sci 115:36–43. https://doi.org/10.1016/j.lfs.2014.09.002
Bulcão RP, Freitas FA, Venturini CG, Dallegrave E, Durgante J, Göethel G, Cerski CTS, Zielinsky P, Pohlmann AR, Guterres SS et al (2013) Acute and subchronic toxicity evaluation of poly(ε-Caprolactone) lipid-core nanocapsules in rats. Toxicol Sci Off J Soc Toxicol 132:162–176. https://doi.org/10.1093/toxsci/kfs334
Deng S, Gigliobianco MR, Censi R, Di Martino P (2020) Polymeric nanocapsules as nanotechnological alternative for drug delivery system: current status, challenges and opportunities. Nanomater Basel Switz. https://doi.org/10.3390/nano10050847
Pohlmann AR, Fonseca FN, Paese K, Detoni CB, Coradini K, Beck RC, Guterres SS (2013) Poly(ϵ-Caprolactone) microcapsules and nanocapsules in drug delivery. Expert Opin Drug Deliv 10:623–638. https://doi.org/10.1517/17425247.2013.769956
Zhang Z, Tsai P-C, Ramezanli T, Michniak-Kohn BB (2013) Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5:205–218. https://doi.org/10.1002/wnan.1211
Jornada DS, Fiel LA, Bueno K, Gerent JF, Petzhold CL, Beck RCR, Guterres SS, Pohlmann AR (2012) Lipid-core nanocapsules: mechanism of self-assembly, control of size and loading capacity. Soft Matter 8:6646–6655. https://doi.org/10.1039/C2SM25754H
dos Menezes PP, Frank LA, dos Lima BS, de Carvalho YMBG, Serafini MR, Quintans-Júnior LJ, Pohlmann AR, Guterres SS, de Araújo AAS (2017) Hesperetin-loaded lipid-core nanocapsules in polyamide: a new textile formulation for topical drug delivery. Int J Nanomedicine 12:2069–2079. https://doi.org/10.2147/IJN.S124564
Toma W, Kyte SL, Bagdas D, Alkhlaif Y, Alsharari SD, Lichtman AH, Chen Z-J, Del Fabbro E, Bigbee JW, Gewirtz DA et al (2017) Effects of paclitaxel on the development of neuropathy and affective behaviors in the mouse. Neuropharmacology 117:305–315. https://doi.org/10.1016/j.neuropharm.2017.02.020
Thomsen R, Christensen MH (2006) MolDock: a new technique for high-accuracy molecular docking. J Med Chem 49:3315–3321. https://doi.org/10.1021/jm051197e
de Araujo AT, dos Passos MP, de Carvalho YMBG, dos Santos LB, de Souza EPBSS, de Souza Araujo AA, Melo MAO, Quintans-Júnior LJ, de Souza Siqueira Quintans J, Guterres SS et al (2020) Linalool-loaded polymeric nanocapsules are a potential candidate to fibromyalgia treatment. AAPS PharmSciTech 21:184. https://doi.org/10.1208/s12249-020-01719-8
Oliveira NK, Frank LA, Squizani ED, Reuwsaat JCV, Marques BM, Motta H, Garcia AWA, Kinskovski UP, Barcellos VA, Schrank A et al (2021) New nanotechnological formulation based on amiodarone-loaded lipid core nanocapsules displays anticryptococcal effect. Eur J Pharm Sci Off J Eur Fed Pharm Sci 162:105816. https://doi.org/10.1016/j.ejps.2021.105816
Pinto CG, Rech VC, Frizzo CP, Hennemman B, Laporta LV, Roden CRB, da Fernandes LS (2017) Development and study of the stability of nanostructured systems containing ginger oil. Discip Sci Nat E Tecnológicas 18:515–529
Conte R, Marturano V, Peluso G, Calarco A, Cerruti P (2017) Recent advances in nanoparticle-mediated delivery of anti-inflammatory phytocompounds. Int J Mol Sci 18:709. https://doi.org/10.3390/ijms18040709
Guterres SS, Alves MP, Pohlmann AR (2007) Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights 2:147–157
Schaffazick SR, Guterres SS, de Freitas LL, Pohlmann AR (2003) Physicochemical characterization and stability of the polymeric nanoparticle systems for drug administration. Quím Nova 26:726–737. https://doi.org/10.1590/S0100-40422003000500017
Boverhof DR, Bramante CM, Butala JH, Clancy SF, Lafranconi M, West J, Gordon SC (2015) Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul Toxicol Pharmacol 73:137–150. https://doi.org/10.1016/j.yrtph.2015.06.001
Chiang C-L, Cheng M-H, Lin C-H (2021) From nanoparticles to cancer nanomedicine: old problems with new solutions. Nanomaterials 11:1727. https://doi.org/10.3390/nano11071727
Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and Regulations. Beilstein J Nanotechnol 9:1050–1074. https://doi.org/10.3762/bjnano.9.98
Ali H, Al-Khalifa AR, Aouf A, Boukhebti H, Farouk A (2020) Effect of nanoencapsulation on volatile constituents, and antioxidant and anticancer activities of algerian origanum glandulosum Desf. essential oil. Sci Rep 10:2812. https://doi.org/10.1038/s41598-020-59686-w
Parris N, Cooke PH, Hicks KB (2005) Encapsulation of essential oils in zein nanospherical particles. J Agric Food Chem 53:4788–4792. https://doi.org/10.1021/jf040492p
Sotelo-Boyás ME, Correa-Pacheco ZN, Bautista-Baños S, Corona-Rangel ML (2017) Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. LWT 77:15–20. https://doi.org/10.1016/j.lwt.2016.11.022
Calvo P, Vila-Jato JL, Alonso MJ (1996) Comparative in vitro evaluation of several colloidal systems, nanoparticles, nanocapsules, and nanoemulsions, as ocular drug carriers. J Pharm Sci 85:530–536. https://doi.org/10.1021/js950474+
Molpeceres J, Aberturas MR, Chacón M, Berges L, Guzmán M (1997) Stability of cyclosporine-loaded poly-X-caprolactone nanoparticles. J Microencapsul 14:777–787. https://doi.org/10.3109/02652049709006828
Mora-Huertas CE, Fessi H, Elaissari A (2010) Polymer-based nanocapsules for drug delivery. Int J Pharm 385:113–142. https://doi.org/10.1016/j.ijpharm.2009.10.018
Clarke S (2013) Development of hierarchical magnetic nanocomposite materials for biomedical applications. Doctoral, Dublin City University
Ajiboye AL, Trivedi V, Mitchell JC (2018) Preparation of polycaprolactone nanoparticles via supercritical carbon dioxide extraction of emulsions. Drug Deliv Transl Res 8:1790–1796. https://doi.org/10.1007/s13346-017-0422-3
Bhattacharjee S (2016) DLS and zeta potential—what they are and what they are not? J Control Release Off J Control Release Soc 235:337–351. https://doi.org/10.1016/j.jconrel.2016.06.017
Patel VR, Agrawal YK (2011) Nanosuspension: an approach to enhance solubility of drugs. J Adv Pharm Technol Res 2:81–87. https://doi.org/10.4103/2231-4040.82950
Barbosa JAC, Abdelsadig MSE, Conway BR, Merchant HA (2019) Using zeta potential to study the ionisation behaviour of polymers employed in modified-release dosage forms and estimating their PKa. Int J Pharm X 1:100024. https://doi.org/10.1016/j.ijpx.2019.100024
Keawchaoon L, Yoksan R (2011) Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf B Biointerfaces 84:163–171. https://doi.org/10.1016/j.colsurfb.2010.12.031
Khoshakhlagh K, Mohebbi M, Koocheki A, Allafchian A (2018) Encapsulation of D-limonene in alyssum homolocarpum seed gum nanocapsules by emulsion electrospraying: morphology characterization and stability assessment. Bioact Carbohydr Diet Fibre 16:43–52. https://doi.org/10.1016/j.bcdf.2018.03.001
Shi F, Zhao Y, Firempong CK, Xu X (2016) Preparation, characterization and pharmacokinetic studies of linalool-loaded nanostructured lipid carriers. Pharm Biol 54:2320–2328. https://doi.org/10.3109/13880209.2016.1155630
Pivetta TP, Simões S, Araújo MM, Carvalho T, Arruda C, Marcato PD (2018) Development of nanoparticles from natural lipids for topical delivery of thymol: investigation of its anti-inflammatory properties. Colloids Surf B Biointerfaces 164:281–290. https://doi.org/10.1016/j.colsurfb.2018.01.053
Staff NP, Grisold A, Grisold W, Windebank AJ (2017) Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol 81:772–781. https://doi.org/10.1002/ana.24951
Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S (2020) Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol 324:113121. https://doi.org/10.1016/j.expneurol.2019.113121
Starobova H, Vetter I (2017) Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci 10:174. https://doi.org/10.3389/fnmol.2017.00174
Stoicea N, Fiorda-Diaz J, Joseph N, Shabsigh M, Arias-Morales C, Gonzalez-Zacarias AA, Mavarez-Martinez A, Marjoribanks S, Bergese SD (2017) Advanced analgesic drug delivery and nanobiotechnology. Drugs 77:1069–1076. https://doi.org/10.1007/s40265-017-0744-y
Frank LA, Contri RV, Beck RCR, Pohlmann AR, Guterres SS (2015) Improving drug biological effects by encapsulation into polymeric nanocapsules. Wiley Interdiscip Rev Nanomed Nanobiotechnol 7:623–639. https://doi.org/10.1002/wnan.1334
Hassan SB, Gali-Muhtasib H, Göransson H, Larsson R (2010) Alpha terpineol: a potential anticancer agent which acts through suppressing NF-KappaB signalling. Anticancer Res 30:1911–1919
Held S, Schieberle P, Somoza V (2007) Characterization of alpha-terpineol as an anti-inflammatory component of orange juice by in vitro studies using oral buccal cells. J Agric Food Chem 55:8040–8046. https://doi.org/10.1021/jf071691m
Ribeiro TP, Porto DL, Menezes CP, Antunes AA, Silva DF, De Sousa DP, Nakao LS, Braga VA, Medeiros IA (2010) Unravelling the cardiovascular effects induced by alpha-terpineol: a role for the nitric oxide-CGMP pathway. Clin Exp Pharmacol Physiol 37:811–816. https://doi.org/10.1111/j.1440-1681.2010.05383.x
Safaripour S, Nemati Y, Parvardeh S, Ghafghazi S, Fouladzadeh A, Moghimi M (2018) Role of L-arginine/SNAP/NO/CGMP/KATP channel signalling pathway in antinociceptive effect of α-terpineol in mice. J Pharm Pharmacol 70:507–515. https://doi.org/10.1111/jphp.12864
Moreira MR, Cruz GMP, Lopes MS, Albuquerque AAC, Leal-Cardoso JH (2001) Effects of terpineol on the compound action potential of the rat sciatic nerve. Braz J Med Biol Res 34:1337–1340. https://doi.org/10.1590/S0100-879X2001001000015
Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J (2005) International union of pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 57:411–425. https://doi.org/10.1124/pr.57.4.5
Schampel A, Kuerten S (2017) Danger: high voltage—the role of voltage-gated calcium channels in central nervous system pathology. Cells. https://doi.org/10.3390/cells6040043
Oz M, Lozon Y, Sultan A, Yang K-HS, Galadari S (2015) Effects of monoterpenes on ion channels of excitable cells. Pharmacol Ther 152:83–97. https://doi.org/10.1016/j.pharmthera.2015.05.006
Park JF, Luo ZD (2010) Calcium channel functions in pain processing. Channels 4:510–517. https://doi.org/10.4161/chan.4.6.12869
Acknowledgements
This research was funded by grants from the Brazilian agencies, National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq), Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES), Foundation to Support Research and Technological Innovation of the State of Sergipe (Fundação de Apoio à Pesquisa e a Inovação Tecnológica do Estado de Sergipe—FAPITEC/SE) and Funder of Studies and Projects (Financiadora de Estudos e Projetos—FINEP). We thank Prof. Dr. Jader Cruz for his collaboration in conducting electrophysiological experiments carried out at the Federal University of Minas Gerais. We also thank teacher Abilio Borghi for the assistance with the English language review.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gouveia, D.N., Guimarães, A.G., Oliveira, M.A. et al. Nanoencapsulated α-terpineol attenuates neuropathic pain induced by chemotherapy through calcium channel modulation. Polym. Bull. 80, 2515–2532 (2023). https://doi.org/10.1007/s00289-022-04161-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04161-x