Abstract
Nanotechnology is a rapidly increasing scientific field due to its immense potential for developing new materials with exceptional structure-dependant characteristics through electrospinning as a feasible technique for producing nanofibers having a wide range of potential applications in cancer detection and diagnostics. Cancer is one of the most deadly diseases that has been afflicting humans for many decades. When dealing with cancer, a regulated and continuous drug release is much desired and effective, because such drugs can cause damage to normal cells. Owing to the high-dose requirements for general anticancer drugs utilized during chemotherapy, they have serious side effects. Anticancer drug-laden nanofibers have been accomplished using nanotechnology for treating cancer, requiring less drug because the drug is stored in these nanofibers for an extended period of time with a tunable drug release profile. Due to the fact that electrospun nanofibers are one of the most advantageous and rapidly evolving products of modern technology, they are promising candidates for cancer therapy. Although numerous research reports have been published on the application of nanofibers, few have focused exclusively on the use of nanofibers in cancer care. Thus, this analysis not only provides a fundamental understanding of the mechanism of electrospinning process and the properties of nanostructured fibrous materials, but also highlights the electrospinning technique's potential as a promising tool for fabricating polymeric nanofibers used for cancer therapy.
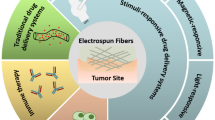
Graphical abstract

Similar content being viewed by others
Abbreviations
- NFs:
-
Nanofibers
References
Pillay V, Dott C, Choonara YE et al (2013) A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J Nanomater. https://doi.org/10.1155/2013/789289
Doshi J, Reneker DH (1995) Electrospinning process and applications of electrospun fibers. J Electrostat 35:151–160. https://doi.org/10.1016/0304-3886(95)00041-8
MacDiarmid AG, Jones WE, Norris ID et al (2001) Electrostatically-generated nanofibers of electronic polymers. Synth Met 119:27–30. https://doi.org/10.1016/S0379-6779(00)00597-X
Reneker DH, Chun I (1996) Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 7:216. https://doi.org/10.1088/0957-4484/7/3/009
Yu J, Qiu Y, Zha X et al (2008) Macromolecular nanotechnology production of aligned helical polymer nanofibers by electrospinning 9:2838–2844. https://doi.org/10.1016/j.eurpolymj.2008.05.020
Kessick R, Tepper G (2004) Microscale polymeric helical structures produced by electrospinning. Appl Phys Lett 84:1182. https://doi.org/10.1063/1.1762704
Bretcanu O, Misra SK, Yunos DM et al (2009) Electrospun nanofibrous biodegradable polyester coatings on Bioglass®-based glass-ceramics for tissue engineering. Mater Chem Phys 118:420–426. https://doi.org/10.1016/j.matchemphys.2009.08.011
Taylor GI (1969) Electrically driven jets. In: Proceedings of the Royal Society of London. A mathematical and physical sciences, vol 313, pp 453–475. https://doi.org/10.1098/RSPA.1969.0205
Cloupeau M, Prunet-Foch B (1990) Electrostatic spraying of liquids: main functioning modes. J Electrostat 25:165–184. https://doi.org/10.1016/0304-3886(90)90025-Q
Grace JM, Marijnissen JCM (1994) A review of liquid atomization by electrical means. J Aerosol Sci 25:1005–1019. https://doi.org/10.1016/0021-8502(94)90198-8
Yarin AL, Koombhongse S, Reneker DH (2001) Bending instability in electrospinning of nanofibers. J Appl Phys 89:3018. https://doi.org/10.1063/1.1333035
Deitzel JM, Kleinmeyer J, Harris D, Beck Tan NC (2001) The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 42:261–272. https://doi.org/10.1016/S0032-3861(00)00250-0
Haider S, Al-Zeghayer Y, Ahmed Ali FA et al (2013) Highly aligned narrow diameter chitosan electrospun nanofibers. J Polym Res. https://doi.org/10.1007/S10965-013-0105-9
Bae HS, Haider A, Selim KMK et al (2013) Fabrication of highly porous PMMA electrospun fibers and their application in the removal of phenol and iodine. J Polym Res. https://doi.org/10.1007/S10965-013-0158-9
Frenot A, Chronakis IS (2003) Polymer nanofibers assembled by electrospinning. Curr Opinion Colloid Interface Sci 8:64–75. https://doi.org/10.1016/S1359-0294(03)00004-9
Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S (2003) A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol 63:2223–2253. https://doi.org/10.1016/S0266-3538(03)00178-7
Ryu YJ, Kim HY, Lee KH et al (2003) Transport properties of electrospun nylon 6 nonwoven mats. Eur Polym J 39:1883–1889. https://doi.org/10.1016/S0014-3057(03)00096-X
Li D, Wang Y, Xia Y (2003) Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett 3:1167–1171. https://doi.org/10.1021/NL0344256
Khajavi R, Abbasipour M (2012) Electrospinning as a versatile method for fabricating coreshell, hollow and porous nanofibers. Sci Iran 19:2029–2034. https://doi.org/10.1016/J.SCIENT.2012.10.037
Chen Z, Foster MD, Zhou W et al (2001) Structure of poly(ferrocenyldimethylsilane) in electrospun nanofibers. Macromolecules 34:6156–6158. https://doi.org/10.1021/MA991857N
Jaeger R, Scho H, Vancso GJ (1996) Chain packing in electro-spun poly(ethylene oxide) visualized by atomic force microscopy
Pedicini A, Farris RJ (2003) Mechanical behavior of electrospun polyurethane. Polymer 44:6857–6862. https://doi.org/10.1016/J.POLYMER.2003.08.040
Yount WC, Loveless DM, Craig SL (2005) Small-molecule dynamics and mechanisms underlying the macroscopic mechanical properties of coordinatively cross-linked polymer networks. J Am Chem Soc 127:14488–14496. https://doi.org/10.1021/JA054298A/SUPPL_FILE/JA054298ASI20050820_022128.PDF
Luo X, Xie C, Wang H et al (2012) Antitumor activities of emulsion electrospun fibers with core loading of hydroxycamptothecin via intratumoral implantation. Int J Pharm 425:19–28. https://doi.org/10.1016/J.IJPHARM.2012.01.012
Cheng S, Du Y, Ma B, Tan D (2009) Total synthesis of a furostan saponin, timosaponin BII. Org Biomol Chem 7:3112–3118. https://doi.org/10.1039/B905091D
Weinberg BD, Blanco E, Gao J (2008) Polymer implants for intratumoral drug delivery and cancer therapy. J Pharm Sci 97:1681–1702. https://doi.org/10.1002/JPS.21038
Long-circulating and target-specific nanoparticles: theory to practice-PubMed. https://pubmed.ncbi.nlm.nih.gov/11356986/. Accessed 25 Dec 2021
Moghimi S, Hunter AC, Murray JC (2001) Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev 53(2):283–318
Lasprilla AJR, Martinez GAR, Lunelli BH et al (2012) Poly-lactic acid synthesis for application in biomedical devices—a review. Biotechnol Adv 30:321–328. https://doi.org/10.1016/J.BIOTECHADV.2011.06.019
Rancan F, Papakostas D, Hadam S et al (2009) Investigation of polylactic acid (PLA) nanoparticles as drug delivery systems for local dermatotherapy. Pharm Res 26:2027–2036. https://doi.org/10.1007/S11095-009-9919-X
EY Gómez-Pachón, R Vera-Graziano, RM Campos 2014 Structure of poly(lactic-acid) PLA nanofibers scaffolds prepared by electrospinning. IOP conference series: materials science and engineering. Vol 59, p 012003 https://doi.org/10.1088/1757-899X/59/1/012003
Zeng J, Yang L, Liang Q et al (2005) Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. J Controll Release 105:43–51. https://doi.org/10.1016/J.JCONREL.2005.02.024
Thangaraju E, Srinivasan NT, Kumar R et al (2012) Fabrication of electrospun poly l-lactide and curcumin loaded poly l-lactide nanofibers for drug delivery. Fibers Polym 13(7):823–830. https://doi.org/10.1007/S12221-012-0823-3
Xu X, Chen X, Xu X et al (2006) BCNU-loaded PEG-PLLA ultrafine fibers and their in vitro antitumor activity against Glioma C6 cells. J Controll Release 114:307–316. https://doi.org/10.1016/J.JCONREL.2006.05.031
Chen P, Wu QS, Ding YP et al (2010) A controlled release system of titanocene dichloride by electrospun fiber and its antitumor activity in vitro. Eur J Pharm Biopharm 76:413–420. https://doi.org/10.1016/J.EJPB.2010.09.005
Zhang Z, Liu S, Qi Y et al (2016) Time-programmed DCA and oxaliplatin release by multilayered nanofiber mats in prevention of local cancer recurrence following surgery. J Controll Release 235:125–133. https://doi.org/10.1016/J.JCONREL.2016.05.046
Qiu K, He C, Feng W et al (2013) Doxorubicin-loaded electrospun poly(l-lactic acid)/mesoporous silica nanoparticles composite nanofibers for potential postsurgical cancer treatment. J Mater Chem B 1:4601–4611. https://doi.org/10.1039/C3TB20636J
Ignatova MG, Manolova NE, Toshkova RA et al (2010) Electrospun nanofibrous mats containing quaternized chitosan and polylactide with in vitro antitumor activity against HeLa cells. Biomacromol 11:1633–1645. https://doi.org/10.1021/BM100285N
Ignatova M, Yossifova L, Gardeva E et al (2011) Antiproliferative activity of nanofibers containing quaternized chitosan and/or doxorubicin against MCF-7 human breast carcinoma cell line by apoptosis. J Bioact Compat Polym 26:539–551. https://doi.org/10.1177/0883911511424655
Toshkova R, Manolova N, Gardeva E et al (2010) Antitumor activity of quaternized chitosan-based electrospun implants against Graffi myeloid tumor. Int J Pharm 400:221–233. https://doi.org/10.1016/J.IJPHARM.2010.08.039
Hasegawa M, Yagi K, Iwakawa S, Hirai M (2001) Chitosan induces apoptosis via caspase-3 activation in bladder tumor cells. Jpn J Cancer Res 92:459–466. https://doi.org/10.1111/J.1349-7006.2001.TB01116.X
Lv G, He F, Wang X et al (2008) Novel nanocomposite of nano Fe3O4 and polylactide nanofibers for application in drug uptake and induction of cell death of leukemia cancer cells. Langmuir 24:2151–2156. https://doi.org/10.1021/LA702845S
Xu X, Chen X, Ma P et al (2008) The release behavior of doxorubicin hydrochloride from medicated fibers prepared by emulsion-electrospinning. Eur J Pharm Biopharm 70:165–170. https://doi.org/10.1016/J.EJPB.2008.03.010
Zhang H, Niu Q, Wang N et al (2015) Thermo-sensitive drug controlled release PLA core/PNIPAM shell fibers fabricated using a combination of electrospinning and UV photo-polymerization. Eur Polym J 71:440–450. https://doi.org/10.1016/J.EURPOLYMJ.2015.08.023
Xie J, Liu W, Macewan MR et al (2014) Neurite outgrowth on electrospun nanofibers with uniaxial alignment: the effects of fiber density, surface coating, and supporting substrate. ACS Nano 8:1878–1885. https://doi.org/10.1021/NN406363J/SUPPL_FILE/NN406363J_SI_003.PDF
Bagó JR, Pegna GJ, Okolie O et al (2016) Electrospun nanofibrous scaffolds increase the efficacy of stem cell-mediated therapy of surgically resected glioblastoma. Biomaterials 90:116–125. https://doi.org/10.1016/j.biomaterials.2016.03.008
Electrospun fibers of poly(l‐lactic acid) containing lovastatin with potential applications in drug delivery. https://en.x-mol.com/paper/article/1234178612260786176. Accessed 25 Dec 2021
Zhu Y, Pyda M, Cebe P (2017) Electrospun fibers of poly(l-lactic acid) containing lovastatin with potential applications in drug delivery. J Appl Polym Sci. https://doi.org/10.1002/APP.45287
Zhang Z, Liu S, Xiong H et al (2015) Electrospun PLA/MWCNTs composite nanofibers for combined chemo—and photothermal therapy. Acta Biomater 26:115–123. https://doi.org/10.1016/J.ACTBIO.2015.08.003
Zhao X, Yuan Z, Yildirimer L et al (2015) Tumor-triggered controlled drug release from electrospun fibers using inorganic caps for inhibiting cancer relapse. Small 11:4284–4291. https://doi.org/10.1002/SMLL.201500985
Xu X, Chen X, Wang Z, Jing X (2009) Ultrafine PEG-PLA fibers loaded with both paclitaxel and doxorubicin hydrochloride and their in vitro cytotoxicity. Eur J Pharm Biopharm 72:18–25. https://doi.org/10.1016/J.EJPB.2008.10.015
Xie J, Wang CH (2006) Electrospun micro—and nanofibers for sustained delivery of paclitaxel to treat C6 Glioma in vitro. Pharm Res 23:1817–1826. https://doi.org/10.1007/S11095-006-9036-Z
Kim K, Luu YK, Chang C et al (2004) Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J Controll Release 98:47–56. https://doi.org/10.1016/J.JCONREL.2004.04.009
Ranganath SH, Fu Y, Arifin DY et al (2010) The use of submicron/nanoscale PLGA implants to deliver paclitaxel with enhanced pharmacokinetics and therapeutic efficacy in intracranial glioblastoma in mice. Biomaterials 31:5199–5207. https://doi.org/10.1016/J.BIOMATERIALS.2010.03.002
Chen Y, Wei J, Hu J et al (2014) Multiple drug-loaded electrospun PLGA/gelatin composite nanofibers encapsulated with mesoporous ZnO nanospheres for potential postsurgical cancer treatment. RSC Adv 4:28011–28019. https://doi.org/10.1039/C4RA03722G
Vashisth P, Kumar N, Sharma M, Pruthi V (2015) Biomedical applications of ferulic acid encapsulated electrospun nanofibers. Biotechnol Rep (Amst) 8:36–44. https://doi.org/10.1016/J.BTRE.2015.08.008
Mehrasa M, Asadollahi MA, Nasri-Nasrabadi B et al (2016) Incorporation of mesoporous silica nanoparticles into random electrospun PLGA and PLGA/gelatin nanofibrous scaffolds enhances mechanical and cell proliferation properties. Mater Sci Eng C Mater Biol Appl 66:25–32. https://doi.org/10.1016/J.MSEC.2016.04.031
Mehrasa M, Asadollahi MA, Ghaedi K et al (2015) Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering. Int J Biol Macromol 79:687–695. https://doi.org/10.1016/J.IJBIOMAC.2015.05.050
Qi RL, Tian XJ, Guo R et al (2016) Controlled release of doxorubicin from electrospun MWCNTs/PLGA hybrid nanofibers. Chin J Polym Sci 34(9):1047–1059. https://doi.org/10.1007/S10118-016-1827-Z
Sasikala ARK, Unnithan AR, Yun YH et al (2016) An implantable smart magnetic nanofiber device for endoscopic hyperthermia treatment and tumor-triggered controlled drug release. Acta Biomater 31:122–133. https://doi.org/10.1016/J.ACTBIO.2015.12.015
Lei C, Cui Y, Zheng L et al (2013) Development of a gene/drug dual delivery system for brain tumor therapy: potent inhibition via RNA interference and synergistic effects. Biomaterials 34:7483–7494. https://doi.org/10.1016/J.BIOMATERIALS.2013.06.010
Chen S, Boda SK, Batra SK et al (2018) Emerging roles of electrospun nanofibers in cancer research. Adv Healthcare Mater 7:e1701024. https://doi.org/10.1002/ADHM.201701024
Natu MV, de Sousa HC, Gil MH (2010) Effects of drug solubility, state and loading on controlled release in bicomponent electrospun fibers. Int J Pharm 397:50–58. https://doi.org/10.1016/J.IJPHARM.2010.06.045
Khor E, Lim LY (2003) Implantable applications of chitin and chitosan. Biomaterials 24:2339–2349. https://doi.org/10.1016/S0142-9612(03)00026-7
Liu X, Lin T, Gao Y et al (2012) Antimicrobial electrospun nanofibers of cellulose acetate and polyester urethane composite for wound dressing. J Biomed Mater Res B Appl Biomater 100:1556–1565. https://doi.org/10.1002/JBM.B.32724
Fischer S, Thümmler K, Volkert B et al (2008) Properties and applications of cellulose acetate. Macromol Symp 262:89–96. https://doi.org/10.1002/MASY.200850210
Assessment UENC for E (2009) Electrospun cellulose acetate fiber mats containing curcumin and release characteristic of the herbal substance
Akaraonye E, Keshavarz T, Roy I (2010) Production of polyhydroxyalkanoates: the future green materials of choice. J Chem Technol Biotechnol 85:732–743. https://doi.org/10.1002/JCTB.2392
(PDF) Polyhydroxyalkanoates: bio-based microbial plastics and their properties. https://www.researchgate.net/publication/228650294_Polyhydroxyalkanoates_Bio-based_microbial_plastics_and_their_properties. Accessed 26 Dec 2021
Peng SW, Guo XY, Shang GG et al (2011) An assessment of the risks of carcinogenicity associated with polyhydroxyalkanoates through an analysis of DNA aneuploid and telomerase activity. Biomaterials 32:2546–2555. https://doi.org/10.1016/J.BIOMATERIALS.2010.12.051
Mottina AC, Ayres E, Orefice RL, Câmara JJD (2016) What changes in Poly(3-Hydroxybutyrate) (PHB) when processed as electrospun nanofibers or thermo-compression molded film? Mater Res 19:57–66. https://doi.org/10.1590/1980-5373-MR-2015-0280
O’Connor S, Szwej E, Nikodinovic-Runic J et al (2013) The anti-cancer activity of a cationic anti-microbial peptide derived from monomers of polyhydroxyalkanoate. Biomaterials 34:2710–2718. https://doi.org/10.1016/j.biomaterials.2012.12.032
Huang C, Soenen SJ, Rejman J et al (2012) Magnetic electrospun fibers for cancer therapy. Adv Func Mater 22:2479–2486. https://doi.org/10.1002/ADFM.201102171
Song M, Guo D, Pan C et al (2008) The application of poly(N-isopropylacrylamide)-co-polystyrene nanofibers as an additive agent to facilitate the cellular uptake of an anticancer drug. Nanotechnology. https://doi.org/10.1088/0957-4484/19/16/165102
Elsner JJ, Zilberman M (2010) Novel antibiotic-eluting wound dressings: an in vitro study and engineering aspects in the dressing’s design. J Tissue Viability 19:54–66. https://doi.org/10.1016/J.JTV.2009.11.001
Han SW, Koh WG (2016) Hydrogel-framed nanofiber matrix integrated with a microfluidic device for fluorescence detection of matrix metalloproteinases-9. Anal Chem 88:6247–6253. https://doi.org/10.1021/ACS.ANALCHEM.5B04867
Abid S, Raza ZA, Rehman A (2016) Synthesis of poly(3-hydroxybutyrate) nanospheres and deposition thereof into porous thin film. Mater Res Express 3:105042. https://doi.org/10.1088/2053-1591/3/10/105042
Zhao Y, Fan Z, Shen M et al (2015) Hyaluronic acid-functionalized electrospun polyvinyl alcohol/polyethyleneimine nanofibers for cancer cell capture applications. Adv Mater Interfaces 2:1500256. https://doi.org/10.1002/admi.201500256
Fan ZY, Zhao YL, Zhu XY et al (2016) Folic acid modified electrospun poly(vinyl alcohol)/polyethyleneimine nanofibers for cancer cell capture applications. Chin J Polym Sci 34:755–765. https://doi.org/10.1007/S10118-016-1792-6
Kumar S, Rai P, Sharma JG et al (2016) PEDOT:PSS/PVA-nanofibers-decorated conducting paper for cancer diagnostics. Adv Mater Technol. https://doi.org/10.1002/ADMT.201600056
Dong J, Chen JF, Smalley M et al (2020) Nanostructured substrates for detection and characterization of circulating rare cells: from materials research to clinical applications. Adv Mater 32:e1903663. https://doi.org/10.1002/ADMA.201903663
Yang G, Wang J, Wang Y et al (2015) An implantable active-targeting micelle-in-nanofiber device for efficient and safe cancer therapy. ACS Nano 9:1161–1174. https://doi.org/10.1021/NN504573U
Barzegar F, Bello A, Fabiane M et al (2015) Preparation and characterization of poly(vinyl alcohol)/graphene nanofibers synthesized by electrospinning. J Phys Chem Solids 77:139–145. https://doi.org/10.1016/J.JPCS.2014.09.015
Wang C, Ma C, Wu Z et al (2015) Enhanced bioavailability and anticancer effect of curcumin-loaded electrospun nanofiber. in vitro and in vivo study. Nanoscale Res Lett 10:1–10. https://doi.org/10.1186/S11671-015-1146-2/FIGURES/8
Zhang N, Deng Y, Tai Q et al (2012) Electrospun TiO2 nanofiber-based cell capture assay for detecting circulating tumor cells from colorectal and gastric cancer patients. Adv Mater 24:2756–2760. https://doi.org/10.1002/ADMA.201200155
Munaweera I, Levesque-Bishop D, Shi Y et al (2014) Radiotherapeutic bandage based on electrospun polyacrylonitrile containing holmium-166 iron garnet nanoparticles for the treatment of skin cancer. ACS Appl Mater Interfaces 6:22250–22256. https://doi.org/10.1021/AM506045K
Jung HS, Kong WH, Sung DK et al (2014) Nanographene oxide-hyaluronic acid conjugate for photothermal ablation therapy of skin cancer. ACS Nano 8:260–268. https://doi.org/10.1021/NN405383A/SUPPL_FILE/NN405383A_SI_001.PDF
Gelain F, Unsworth LD, Zhang S (2010) Slow and sustained release of active cytokines from self-assembling peptide scaffolds. J Controll Release 145:231–239. https://doi.org/10.1016/J.JCONREL.2010.04.026
Sridhar R, Ravanan S, Venugopal JR et al (2014) Curcumin- and natural extract-loaded nanofibres for potential treatment of lung and breast cancer: in vitro efficacy evaluation. J Biomater Sci Polym Ed 25:985–998. https://doi.org/10.1080/09205063.2014.917039
Yohe ST, Herrera VLM, Colson YL, Grinstaff MW (2012) 3D superhydrophobic electrospun meshes as reinforcement materials for sustained local drug delivery against colorectal cancer cells. J Controll Release 162:92–101. https://doi.org/10.1016/J.JCONREL.2012.05.047
Yohe ST, Colson YL, Grinstaff MW (2012) Superhydrophobic materials for tunable drug release: using displacement of air to control delivery rates. J Am Chem Soc 134:2016–2019. https://doi.org/10.1021/JA211148A
Fu Y, Li X, Ren Z et al (2018) Multifunctional electrospun nanofibers for enhancing localized cancer treatment. Small 14:e1801183. https://doi.org/10.1002/SMLL.201801183
Shao S, Li L, Yang G et al (2011) Controlled green tea polyphenols release from electrospun PCL/MWCNTs composite nanofibers. Int J Pharm 421:310–320. https://doi.org/10.1016/J.IJPHARM.2011.09.033
Jiang J, Xie J, Ma B et al (2014) Mussel-inspired protein-mediated surface functionalization of electrospun nanofibers for pH-responsive drug delivery. Acta Biomater 10:1324–1332. https://doi.org/10.1016/J.ACTBIO.2013.11.012
Xue R, Behera P, Xu J et al (2014) Polydimethylsiloxane core-polycaprolactone shell nanofibers as biocompatible, real-time oxygen sensors. Sens Actuators B Chem 192:697. https://doi.org/10.1016/J.SNB.2013.10.084
Agudelo-Garcia PA, de Jesus JK, Williams SP et al (2011) Glioma cell migration on three-dimensional nanofiber scaffolds is regulated by substrate topography and abolished by inhibition of STAT3 signaling. Neoplasia 13:831–840. https://doi.org/10.1593/NEO.11612
Sims-Mourtada J, Niamat RA, Samuel S et al (2014) Enrichment of breast cancer stem-like cells by growth on electrospun polycaprolactone-chitosan nanofiber scaffolds. Int J Nanomed 9:995. https://doi.org/10.2147/IJN.S55720
Jain A, Betancur M, Patel GD et al (2014) Guiding intracortical brain tumour cells to an extracortical cytotoxic hydrogel using aligned polymeric nanofibres. Nat Mater 13:308–316. https://doi.org/10.1038/NMAT3878
Hartman O, Zhang C, Adams EL et al (2010) Biofunctionalization of electrospun PCL-based scaffolds with perlecan domain IV peptide to create a 3-D pharmacokinetic cancer model. Biomaterials 31:5700–5718. https://doi.org/10.1016/J.BIOMATERIALS.2010.03.017
Hesami P, Holzapfel BM, Taubenberger A et al (2014) A humanized tissue-engineered in vivo model to dissect interactions between human prostate cancer cells and human bone. Clin Exp Metas 31:435–446. https://doi.org/10.1007/S10585-014-9638-5
Ravanan S, Prema AA, Xavier RJ, Sahayaraj PA (2016) Anti-cancer activity (A431 cancer cells) and cytotoxic efficiency (HaCaT skin cells) of Curcumin/Neem loaded polycaprolactone (PCL) nanofibres. Der Pharma Chem 8:104–111
Lin WC, Yeh IT, Niyama E et al (2018) Electrospun poly (ε-caprolactone) nanofibrous mesh for imiquimod delivery in melanoma therapy. Polymers 10:231. https://doi.org/10.3390/POLYM10030231
Garrett R, Niiyama E, Kotsuchibashi Y et al (2015) Biodegradable nanofiber for delivery of immunomodulating agent in the treatment of basal cell carcinoma. Fibers 3:478–490. https://doi.org/10.3390/FIB3040478
Ramírez-Agudelo R, Scheuermann K, Gala-García A et al (2018) Hybrid nanofibers based on poly-caprolactone/gelatin/hydroxyapatite nanoparticles-loaded Doxycycline: Effective anti-tumoral and antibacterial activity. Mater Sci Eng C Mater Biol Appl 83:25–34. https://doi.org/10.1016/J.MSEC.2017.08.012
Zhu LF, Zheng Y, Fan J et al (2019) A novel core-shell nanofiber drug delivery system intended for the synergistic treatment of melanoma. Eur J Pharm Sci. https://doi.org/10.1016/J.EJPS.2019.105002
Janani I, Lakra R, Kiran MS, Korrapati PS (2018) Selectivity and sensitivity of molybdenum oxide-polycaprolactone nanofiber composites on skin cancer: Preliminary in-vitro and in-vivo implications. J Trace Elements Med Biol 49:60–71. https://doi.org/10.1016/J.JTEMB.2018.04.033
Nakadaira H, Endoh K, Yamamoto M, Katoh K (1995) Distribution of selenium and molybdenum and cancer mortality in Niigata, Japan. Arch Environ Health 50:374–380. https://doi.org/10.1080/00039896.1995.9935970
Xi Y, Ge J, Guo Y et al (2018) Biomimetic elastomeric polypeptide-based nanofibrous matrix for overcoming multidrug-resistant bacteria and enhancing full-thickness wound healing/skin regeneration. ACS Nano 12:10772–10784. https://doi.org/10.1021/ACSNANO.8B01152/SUPPL_FILE/NN8B01152_SI_001.PDF
Yu DG, Li XY, Wang X et al (2015) Nanofibers fabricated using triaxial electrospinning as zero order drug delivery systems. ACS Appl Mater Interfaces 7:18891–18897. https://doi.org/10.1021/ACSAMI.5B06007/SUPPL_FILE/AM5B06007_SI_001.PDF
Jayakumar R, Prabaharan M, Nair SV, Tamura H (2010) Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol Adv 28:142–150. https://doi.org/10.1016/J.BIOTECHADV.2009.11.001
Ignatova M, Manolova N, Rashkov I (2007) Novel antibacterial fibers of quaternized chitosan and poly(vinyl pyrrolidone) prepared by electrospinning. Eur Polym J 43:1112–1122. https://doi.org/10.1016/J.EURPOLYMJ.2007.01.012
Ma G, Liu Y, Peng C et al (2011) Paclitaxel loaded electrospun porous nanofibers as mat potential application for chemotherapy against prostate cancer. Carbohyd Polym 86:505–512. https://doi.org/10.1016/J.CARBPOL.2011.04.082
Ardeshirzadeh B, Anaraki NA, Irani M et al (2015) Controlled release of doxorubicin from electrospun PEO/chitosan/graphene oxide nanocomposite nanofibrous scaffolds. Mater Sci Eng C Mater Biol Appl 48:384–390. https://doi.org/10.1016/J.MSEC.2014.12.039
Li W, Luo T, Yang Y et al (2015) Formation of controllable hydrophilic/hydrophobic drug delivery systems by electrospinning of vesicles. Langmuir 31:5141–5146. https://doi.org/10.1021/LA504796V
Achille C, Sundaresh S, Chu B, Hadjiargyrou M (2012) Cdk2 silencing via a DNA/PCL electrospun scaffold suppresses proliferation and increases death of breast cancer cells. PLoS ONE 7:e52356. https://doi.org/10.1371/JOURNAL.PONE.0052356
Radmansouri M, Bahmani E, Sarikhani E et al (2018) Doxorubicin hydrochloride—Loaded electrospun chitosan/cobalt ferrite/titanium oxide nanofibers for hyperthermic tumor cell treatment and controlled drug release. Int J Biol Macromol 116:378–384. https://doi.org/10.1016/J.IJBIOMAC.2018.04.161
Lin TC, Lin FH, Lin JC (2012) In vitro feasibility study of the use of a magnetic electrospun chitosan nanofiber composite for hyperthermia treatment of tumor cells. Acta Biomater 8:2704–2711. https://doi.org/10.1016/J.ACTBIO.2012.03.045
Park Y, Kang E, Kwon OJ et al (2010) Ionically crosslinked Ad/chitosan nanocomplexes processed by electrospinning for targeted cancer gene therapy. J Controll Release 148:75–82. https://doi.org/10.1016/J.JCONREL.2010.06.027
Yang C, Chu L, Zhang Y et al (2015) Dynamic biostability, biodistribution, and toxicity of L/D-peptide-based supramolecular nanofibers. ACS Appl Mater Interfaces 7:2735–2744. https://doi.org/10.1021/AM507800E
Krishnan R, Sundarrajan S, Ramakrishna S (2013) Green processing of nanofibers for regenerative medicine. Macromol Mater Eng 298:1034–1058. https://doi.org/10.1002/MAME.201200323
Poláková L, Širc J, Hobzová R et al (2019) Electrospun nanofibers for local anticancer therapy: review of in vivo activity. Int J Pharm 558:268–283. https://doi.org/10.1016/J.IJPHARM.2018.12.059
Tort S, Han D, Steckl AJ (2020) Self-inflating floating nanofiber membranes for controlled drug delivery. Int J Pharm 579:119164. https://doi.org/10.1016/J.IJPHARM.2020.119164
Garkal A, Kulkarni D, Musale S et al (2021) Electrospinning nanofiber technology: a multifaceted paradigm in biomedical applications. New J Chem 45:21508–21533. https://doi.org/10.1039/D1NJ04159B
Dziemidowicz K, Sang Q, Wu J et al (2021) Electrospinning for healthcare: recent advancements. J Mater Chem B 9:939–951. https://doi.org/10.1039/D0TB02124E
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jagtiani, E., Sabnis, A.S. Recent advancements of electrospun nanofibers for cancer therapy. Polym. Bull. 80, 1215–1242 (2023). https://doi.org/10.1007/s00289-022-04153-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04153-x