Abstract
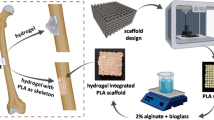
When manufacturing scaffolds that will be used in tissue engineering of the bone system, three properties need to be considered: (1) The biodegradation times. (2) The mechanical properties and structure of the scaffold must emulate that of the bone. (3) The biocompatibility of the scaffold with bone cells. In this work, a scaffold is obtained in the shape of a rabbit tibia using a 3D-printer with commercial polycaprolactone of medium molecular mass. Then, the scaffold is covered by electrospinning with a layer of synthesized polycaprolactone of low molecular mass. 3D-printing mimics the tissue structure in a rabbit tibia bone. Polycaprolactone is hydrophobic polymer that stops cells from adhering to the scaffold. To improve cell adhesion, gelatin molecules were grafted onto the polycaprolactone. The scaffolds were characterized with FTIR, SEM, capillary viscometry, DSC, contact angle, accelerated degradation, and DMA. The cytotoxic evaluation was carried out on scaffolds in which we used human adipose stem cells, the addition of gelatin improved cell viability. The results of the cytotoxic tests showed viability and proliferation of cells when they were seeded on the scaffolds and were evaluated on different days. Additionally, an evaluation was conducted to determine whether the scaffolds allowed for the differentiation of cells into an osteogenic lineage, when the cells were cultured with an osteoinductive medium. At 21 days, the presence of calcium nodules stained with Alizarin Red and black nodules of calcium phosphate particles stained with von Kossa was demonstrated.













Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Abbreviations
- vPCLc:
-
Commercial virgin polycaprolactone
- vPCLs:
-
Virgin polycaprolactone synthesized in our laboratory
- 3D-PCL:
-
3D-printed scaffold of commercial polycaprolactone
- bPCL:
-
Electrospun/3D-printed scaffold of synthesized/commercial polycaprolactone
- bPCLg:
-
Electrospun/3D-printed scaffold of synthesized/commercial polycaprolactone grafted with gelatin
References
Lanza R, Langer R, Vacanti J, Atala A (eds) (2020) Principles of tissue engineering. Academic Press, USA. https://doi.org/10.1016/C2018-0-03818-9
Szczepańczyk P, Szlachta M, Złocista-Szewczyk N, Chłopek J, Pielichowska K (2021) Recent developments in polyurethane-based materials for bone tissue engineering. Polymers 13(6):946. https://doi.org/10.3390/polym13060946
Preethi Soundarya S, Haritha Menon A, Viji Chandran S, Selvamurugan N (2018) Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int J Biol Macromol 119:1228–1239. https://doi.org/10.1016/j.ijbiomac.2018.08.056
Chuc-Gamboa MG, Cámara Perera CM, Aguilar-Ayala FJ, Vargas-Coronado RF, Cauich-Rodríguez JV, Escobar-García DM, Sánchez-Vargas LO, Pacheco N, San Roman J (2021) Antibacterial behavior of chitosan-sodium hyaluronate-PEGDE crosslinked films. Appl Sci 11:1267. https://doi.org/10.3390/app11031267
Wubneh A, Tsekoura EK, Ayranci C, Uludag H (2018) Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater 80:1–30. https://doi.org/10.1016/j.actbio.2018.09.031
Deb S, Mandegaran R, Di Silvio L (2010) A porous scaffold for bone tissue engineering/45S5 Bioglass® derived porous scaffolds for co-culturing osteoblasts and endothelial cells. J Mater Sci: Mater Med 21:893–905. https://doi.org/10.1007/s10856-009-3936-5
Aguilar-Pérez FJ, Vargas-Coronado RF, Cervantes-Uc JM, Cauich-Rodríguez JV, Rosales-Ibáñez R, Rodríguez-Ortiz JA, Torres-Hernández Y (2019) Titanium-castor oil based polyurethane composite foams for bone tissue engineering. J Biomater Sci Polym Ed 30(15):1415–1432. https://doi.org/10.1080/09205063.2019.1636352
Das D, Pandey I, Chakraborty A, Banerjee JS (2016) Analysis of implementation factors of 3D printer: the key enabling technology for making prototypes of the engineering design and manufacturing. Int J Comput Appl. https://www.ijcaonline.org/proceedings/micro2016/number1/28436-6102
Tylek T, Blum C, Hrynevich A, Schlegelmilch T, Dalton PD, Groll J (2020) Precisely defined fiber scaffolds with 40 μm porosity induce elongation driven M2-like polarization of human macrophages. Biofabrication 12:2. https://doi.org/10.1088/1758-5090/ab5f4e
Ligon SC, Liska R, Stampfl J, Gurr M, Mülhaupt R (2017) Polymers for 3D printing and customized additive manufacturing. Chem Rev 117:0212–10290. https://doi.org/10.1021/acs.chemrev.7b00074
Long YZ, Yan X, Wang XX, Zhang J, Yu M (2019) Electrospinning: the setup and procedure. In: Ding B, Wang X, Yu J (eds) Micro and nano technologies electrospinning: nanofabrication and applications. William Andrew Publishing, pp 21–52
Sánchez-Pech JC, Rosales-Ibáñez R, Cauich-Rodríguez JV, Carrillo-Escalante HJ, Rodríguez-Navarrete A, Ávila-Ortega A, Hernández-Sánchez F (2020) Design, synthesis, characterization, and cytotoxicity of PCL/PLGA scaffolds through plasma treatment in the presence of pyrrole for possible use in urethral tissue engineering. J Biomater Appl 34(6):840–850. https://doi.org/10.1177/0885328219882638
Yu Y, Hua S, Yang M, Fu Z, Teng S, Niu K, Zhao Q, Yi C (2016) Fabrication and characterization of electrospinning/3D printing bone tissue engineering scaffold. RSC Adv. https://doi.org/10.1039/c6ra17718b
Huang B, Aslan E, Jiang Z, Daskalakis E, Jiao M, Aldalbahi A, Vyas C, Bártolo P (2020) Engineered dual-scale poly(ecaprolactone) scaffolds using 3D printing and rotacional electrospinning for bone tissue regeneration. Addit Manuf. https://doi.org/10.1016/j.addma.2020.101452
MaShiSuWang QKTZ (2020) Biodegradation of polycaprolactone (PCL) with different molecular weights by Candida antarctica lipase. J Polym Environ 28:2947–2955. https://doi.org/10.1007/s10924-020-01826-4
Gorrasi G, Pappalardo D, Pellecchia C (2012) Polymerization of ε-caprolactone by sodium hydride: from the synthesis of the polymer samples to their thermal, mechanical and barrier properties. React Funct Polym 72(10):752–756. https://doi.org/10.1016/j.reactfunctpolym.2012.07.007
Grodzinski JJ (2006) Polymers for tissue engineering, medical devices, and regenerative medicine. Concise general review of recent studies. Polym Adv Technol 17(1):474–478. https://doi.org/10.1002/pat.729
Hong JK, Xu G, Piao D, Madihally SV (2012) Analysis of void share and size in the collector plate and polycaprolactone molecular weight on electrospun scaffold pore Size. J Appl Polym Sci 128(3):1583–1591. https://doi.org/10.1002/app.38326
Goddard JM, Hotchkiss JH (2007) Polymer surface modification for the attachment of bioactive compounds. Prog Polym Sci 32(7):698–725. https://doi.org/10.1016/j.progpolymsci.2007.04.002
Gregory CA, Gunn WG, Peister A, Prockop DJ (2004) An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem 329(1):77–84. https://doi.org/10.1016/j.ab.2004.02.002
Delorme B, Charbord P (2007) Culture and characterization of human bone marrow mesenchymal stem cells. Methods Mol Med 140:67–81. https://doi.org/10.1007/978-1-59745-443-8_4
Nguyen NT, Thurecht KJ, Howdle SM, Irvine DJ (2014) Facile one-spot synthesis of highly branched polycaprolactone. Polym Chem 5(8):2997–3008. https://doi.org/10.1039/C3PY01725G
Heuschen J, Jerome R, Teyssie P (1981) Polycaprolactone-based block copolymers. 1. Synthesis by anionic coordination type catalysts. Macromolecules 14(2):242–246. https://doi.org/10.1021/ma50003a003
Huang YP, Xu X, Luo XL, Ma DZ (2002) Molecular weight dependence of the melting behavior of oly (ε-caprolactone). Chin Polym Sci 20(1):45–51
Zhu Y, Gao C, Liu X, Shen J (2002) Surface modification of polycaprolactone membrane via aminolysis and biomacromolecule immobilization for promoting cytocompatibility of human endothelial cells. Biomacromol 3:1312–1319. https://doi.org/10.1021/bm020074y
Hu Y, Feng B, Zhang W, Yan C, Yao Q, Shao C, Yu F, Li F, Fu Y (2019) Electrospun gelatin/PCL and collagen/PCL scaffolds for modulating responses of bone marrow endothelial progenitor cells. Exp Ther Med 17(5):3717–3726. https://doi.org/10.3892/etm.2019.7387
Gentile P, McColgan-Bannon K, Ceretto Gianone N, Sefat F, Dalgarmo K, Ferreira AM (2017) Biosynthetic PCL-graft-collagen bulk material for tissue engineering applications. Materials 2(693):1–17. https://doi.org/10.3390/ma10070693
Wen XX, Xu C, Wang FQ, Feng YF, Zhao X, Yan YB, Lei W (2015) Temporal changes of microarchitectural and mechanical parameters of cancellous bone in the osteoporotic rabbit. Biomed Res Int 2015:11. https://doi.org/10.1155/2015/263434
Martín-de León J, Bernardo V, Rodríguez-Pérez MA (2019) Nanocellular polymers: the challenge of creating cells in the nanoscale. Materials 12(5):797. https://doi.org/10.3390/ma12050797
Valadez-González A, Rosales-Ibáñez R, Rodríguez-Navarrete A, Villamar-Duque TE, Cano-BrownOrtiz-Fernánde C-EA, Hernández-Sánchez F (2021) Tailoring surface properties of carbon nanofibers via oxidation and its influence on dental pulp stem cell viability of PCL/CNF composites. Polym Bull 78:695–711. https://doi.org/10.1007/s00289-020-03127-1
de la Vega OA, Bonilla Ríos J, Ramos de Valle LF, de Almeida Prado LAS, Schulte K (2007) Peroxide assisted coupling and characterization of carbon-nanofiber-reinforced poly(propylene) composites macromol. Mater Eng 292:1095–1102. https://doi.org/10.1002/mame.200700201
Orozco-CastellanosMarcos-FernándezMartínez-Richa LMAA (2011) Hydrolytic degradation of poly(ε-caprolactone) with different end groups and poly(ε-caprolactone-co-γ-butyrolactone): characterization and kinetics of hydrocortisone delivery. Polym Adv Technol 22(4):430–436. https://doi.org/10.1002/pat.1531
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 28(4):315–7. https://doi.org/10.1080/14653240600855905
Sattary M, Rafienia M, Kazemi M, Salehi H, Mahmoudzadeh M (2019) Promoting effect of nano hydroxyapatite and vitamin D3 on the osteogenic differentiation of human adipose-derived stem cells in polycaprolactone/gelatin scaffold for bone tissue engineering. Mater Sci Eng C Mater Biol Appl 97:141–155. https://doi.org/10.1016/j.msec.2018.12.030
Samadian H, Farzamfar VA, Ehterami A, Bit A, Alam M, Goodarzi A, Darya G, Salehi M (2020) A tailored polylactic acid/polycaprolactone biodegradable and bioactive 3D porous scaffold containing gelatin nanofibers and Taurine for bone regeneration. Sci Rep 10:13366. https://doi.org/10.1038/s41598-020-70155-2
Acknowledgements
Special thanks to Dr. José Tapia Ramírez for his hASCs donation from CINVESTAV-Zacatenco, Mexico City, A. Rodríguez-Navarrete from UNAM for her participation in biological analysis, Eng. Silvia Andrade from CICY for her participation in Scanning Electron Microscope, and Dr. Nancy ArandaCirerol for her recommendations in the editing manuscript.
Funding
This project was supported by Basic Science Project CONACyT No. 283972, Master Scholarship CONACyT 705198, Support Program for Research and Technological Innovation Projects (Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica, PAPIIT; in Spanish) UNAM IA207420.
Author information
Authors and Affiliations
Contributions
H-S, R-I and C-R conceptualized the work; V-R, C-E, G-G, and R-M curated data; H-S, R-I, C-E, G-G, and R-M conducted formal analysis; R-I acquired fund; H-S, C-R, and R-I conducted investigation; V-R, G-G, and R-M were responsible for the methodology; H-S, C-R, and R-I supervised the work; H-S, C-R, and R-I wrote the original draft and reviewed and edited the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rosales-Ibáñez, R., Viera-Ruiz, A.E., Cauich-Rodríguez, J.V. et al. Electrospun/3D-printed PCL bioactive scaffold for bone regeneration. Polym. Bull. 80, 2533–2552 (2023). https://doi.org/10.1007/s00289-022-04149-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04149-7