Abstract
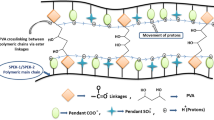
Polyelectrolytic membranes, such as the well-known commercial grade Nafion™, suffer mechanical failure and chemical decomposition after many hours of operation into a fuel cell. Mechanically due to expansion–contraction phenomena during water management and chemically due to the hydrogen peroxide and radicals produced from the H2/O2 redox electrochemical reactions. Considering that such behavior exists for the most used and reliable Nafion™ membranes, it is also expected similar properties depletion for alternative membrane copolymers. In this work, we examined the performance of sulfonated Styrene/butyl acrylate (S-St-BuA) copolymers during proton exchange activity through redox electrochemical reactions and their interactions with Fenton’s reagent (H2O2/FeCl2), compared with Nafion™. In situ, FTIR is a simultaneous analysis that evaluates the spectroscopic characteristics while the proton exchange into membranes is also measured. The in situ FTIR analysis showed chemical stability in the structure of the alternative membranes of S-St/BuA, compared with Nafion™ when both were exposed to Fenton's reagent.









Similar content being viewed by others
References
Grunwaldt J-D, Baiker A (2005) In situ spectroscopic investigation of heterogeneous catalysts and reaction media at high pressure. Phys Chem Chem Phys 7:3526–3539. https://doi.org/10.1039/B509667G
Iwasita T, Nart FC (1997) In situ infrared spectroscopy at electrochemical interfaces. Prog Surf Sci 55:271–340. https://doi.org/10.1016/S0079-6816(97)00032-4
Rice C, Tong OE, Wieckowski A, Hahn F, Gloaguen F et al (2000) In situ infrared study of carbon monoxide adsorbed onto commercial fuel-cell-grade carbon-supported platinum nanoparticles: correlation with 13C NMR results. J Phys Chem B 104:5803–5807. https://doi.org/10.1021/jp0007179
Ye J-Y, Jiang Y-X, Sheng T, Sun S-G (2016) In-situ FTIR spectroscopic studies of electrocatalytic reactions and processes. Nano Energy 29:414–427. https://doi.org/10.1016/j.nanoen.2016.06.023
Bewick A (1983) In-situ infrared spectroscopy of the electrode/electrolyte solution interphase. J Electroanal Chem Interfacial Electrochem 150:481–493. https://doi.org/10.1016/S0022-0728(83)80228-9
Amenabar I, Poly S, Nuansing W, Hubrich EH, Govyadinov AA, Huth F et al (2013) Structural analysis and mapping of individual protein complexes by infrared nanospectroscopy. Nat Commun 4:2890. https://doi.org/10.1038/ncomms3890
Coenen K, Gallucci F, Mezari B, Hensen E, van Sint Annaland M (2018) An in-situ IR study on the adsorption of CO2 and H2O on hydrotalcites. J CO2 Util 24:228–239. https://doi.org/10.1016/j.jcou.2018.01.008
Ogihara Y, Yano H, Matsumoto T, Tryk DA, Iiyama A, Uchida H (2017) In situ FTIR analysis of CO-tolerance of a Pt-Fe alloy with stabilized Pt skin layers as a hydrogen anode catalyst for polymer electrolyte fuel cells. Catal 7:8. https://doi.org/10.3390/catal7010008
Zhao X, Mao L, Dong G (2018) Mn-Ce-V-WOx/TiO2 SCR catalysts: catalytic activity, stability and interaction among catalytic oxides. Catal. https://doi.org/10.3390/catal8020076
Nakamura R, Imanishi A, Murakoshi K, Nakato Y (2003) In situ FTIR studies of primary intermediates of photocatalytic reactions on nanocrystalline TiO2 films in contact with aqueous solutions. J Am Chem Soc 125:7443–7450. https://doi.org/10.1021/ja029503q
Ayato Y, Kunimatsu K, Osawa M, Okada T (2006) Study of Pt electrode/nafion ionomer interface in HClO[sub 4] by in situ surface-enhanced FTIR spectroscopy. J Electrochem Soc 153:A203. https://doi.org/10.1149/1.2137648
Tkach I, Panchenko A, Kaz T, Gogel V, Friedrich KA, Roduner E (2004) In situ study of methanol oxidation on Pt and Pt/Ru-mixed with Nafion® anodes in a direct methanol fuel cell by means of FTIR spectroscopy. Phys Chem Chem Phys 6:5419–5426. https://doi.org/10.1039/B411108G
Falk M (2011) An infrared study of water in perfluorosulfonate (Nafion) membranes. Can J Chem 58:1495–1501. https://doi.org/10.1139/v80-237
Fernandes AC, Ticianelli EA (2009) A performance and degradation study of Nafion 212 membrane for proton exchange membrane fuel cells. J Power Sources 193:547–554. https://doi.org/10.1016/j.jpowsour.2009.04.038
Yu TH, Sha Y, Liu W-G, Merinov BV, Shirvanian P, Goddard WA (2011) Mechanism for degradation of nafion in PEM fuel cells from quantum mechanics calculations. J Am Chem Soc 133:19857–19863. https://doi.org/10.1021/ja2074642
Ghassemzadeh L, Kreuer KD, Maier J, Müller K (2011) Evaluating chemical degradation of proton conducting perfluorosulfonic acid ionomers in a Fenton test by solid-state 19F NMR spectroscopy. J Power Sources 196:2490–2497. https://doi.org/10.1016/j.jpowsour.2010.11.053
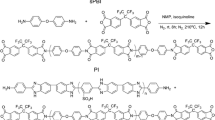
Francisco-Vieira L, Benavides R, Cuara-Diaz E, Morales-Acosta D (2019) Styrene-co-butyl acrylate copolymers with potential application as membranes in PEM fuel cell. Int J Hydrog Energy 44:12492–12499. https://doi.org/10.1016/J.IJHYDENE.2019.01.181
Lu G-Q, Sun S-G, Cai L-R, Chen S-P, Tian Z-W, Shiu K-K (2000) In situ FTIR spectroscopic studies of adsorption of CO, SCN-, and poly(o-phenylenediamine) on electrodes of nanometer thin films of Pt, Pd, and Rh: abnormal infrared effects (AIREs). Langmuir 16:778–786. https://doi.org/10.1021/la990282k
Mojet BL, Ebbesen SD, Lefferts L (2010) Light at the interface: the potential of attenuated total reflection infrared spectroscopy for understanding heterogeneous catalysis in water. Chem Soc Rev 39:4643–4655. https://doi.org/10.1039/C0CS00014K
Singh RK, Kunimatsu K, Miyatake K, Tsuneda T (2016) Experimental and theoretical infrared spectroscopic study on hydrated nafion membrane. Macromol 49:6621–6629. https://doi.org/10.1021/acs.macromol.6b00999
Acknowledgments
To CONACyT-México for the Project FC3004 and Post-Doctoral Grant for AASC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Siller-Ceniceros, A.A., Benavides, R., Francisco-Vieira, L. et al. Chemical stability of polyelectrolyte sulfonated membranes (St-BuA) in acid media: simultaneous electrochemical and spectroscopic characterization by in-situ FTIR. Polym. Bull. 80, 1399–1410 (2023). https://doi.org/10.1007/s00289-022-04117-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04117-1