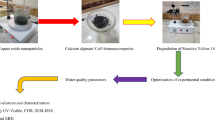
Abstract
Wastewater contaminated by dye produced by the rapid industrialization is a global problem. One type of the dyes used in the industry is malachite green (MG). In this study, we reported the results of designing and synthesizing the Ca-Alginate (Metal Organic Framework (MOF)) as well as its application as an adsorbent to reduce the amount of MG in wastewater. The alginate used for the synthesis of Ca-Alginate was first obtained by extracting it from brown algae using sodium carbonate as a solvent. In this study, Ca-Alginate was obtained by ion exchange process using calcium chloride solution and calcination at a temperature of 900 °C. Based on the characterization data, it was found that the adsorbent has a macropore structure with a specific surface area of 0.614 m2/g and a total pore volume of 1.744 × 10−3 cc/g. The result of the FTIR characterization showed the presence of hydroxyl groups, symmetrical and asymmetrical carboxyl groups, and mannuronic and guluronic groups. These groups indicated the presence of alginate. The adsorption ability test on the MG dye was performed at various changes in contact time and concentration of the adsorbent, while the MG concentration on the adsorption process was examined using the batch system. The results showed that the highest reduction in MG occurred at the contact time of 120 min, 0.06 g/L of adsorbent dosage and 0.0055 g/L of initial MG concentration. Under these conditions the Ca-Alginate could adsorb up to 84.47% of MG. The adsorption kinetic of MG onto Ca-Alginate obeyed the modified pseudo-first-order (MPFO) model.
Graphical abstract







Similar content being viewed by others
References
Abbasi M, Sabzehmeidani MM, Ghaedi M, Jannesar R, Shokrollahi A (2021) Synthesis of grass-like structured Mn–Fe layered double hydroxides/PES composite adsorptive membrane for removal of malachite green. Appl Clay Sci 203:105946. https://doi.org/10.1016/j.clay.2020.105946
Abdelrahman EA (2018) Synthesis of zeolite nanostructures from waste aluminum cans for efficient removal of malachite green dye from aqueous media. J Mol Liquids 253:72–82. https://doi.org/10.1016/j.molliq.2018.01.038
Akram M, Bhatti HN, Iqbal M, Noreen S, Sadaf S (2017) Biocomposite efficiency for Cr(VI) adsorption: kinetic, equilibrium and thermodynamics studies. J Environ Chem Eng 5(1):400–411. https://doi.org/10.1016/j.jece.2016.12.002
Amiri M, Salavati-Niasari M, Akbari A, Gholami T (2017) Removal of malachite green (a toxic dye) from water by cobalt ferrite silica magnetic nanocomposite: herbal and green sol-gel autocombustion synthesis. Int J Hydrogen Energy 42(39):24846–24860. https://doi.org/10.1016/j.ijhydene.2017.08.077
Azizian S, Fallah RN (2010) A new empirical rate equation for adsorption kinetics at solid/solution interface. Appl Surf Sci 256(17):5153–5156. https://doi.org/10.1016/j.apsusc.2009.12.080
Bua P (2020) Makalah Kimia Dasar 2 (Kimia Unsur). Universitas Negeri Gorontalo, Gorontalo
Budiana IGMN, Jasman J, Neolaka YAB, Riwu AAP, Elmsellem H, Darmokoesoemo H, Kusuma HS (2021) Synthesis, characterization and application of cinnamoyl C-phenylcalix[4]resorcinarene (CCPCR) for removal of Cr(III) ion from the aquatic environment. J Mol Liquids 324:114776. https://doi.org/10.1016/j.molliq.2020.114776
Cardenas-Jiron G, Leal D, Matsuhiro B, Osorio-Roman IO (2011) Vibrational spectroscopy and density functional theory calculations of poly-D-mannuronate and heteropolymeric fractions from sodium alginate. J Raman Spectrosc 42(4):870–878. https://doi.org/10.1002/jrs.2760
Chandia NP, Matsuhiro B, Vasquez AE (2001) Alginic acids in Lessonia trabeculata: characterization by formic acid hydrolysis and FT-IR spectroscopy. Carbohyd Polym 46(1):81–87. https://doi.org/10.1016/S0144-8617(00)00286-1
Cui H, Li Q, Qian Y, Tang R, An H, Zhai J (2011) Defluoridation of water via electrically controlled anion exchange by polyaniline modified electrode reactor. Water Res 45(17):5736–5744. https://doi.org/10.1016/j.watres.2011.08.049
Daradmare S, Xia M, Le VN, Kim J, Park BJ (2021) Metal–organic frameworks/alginate composite beads as effective adsorbents for the removal of hexavalent chromium from aqueous solution. Chemosphere 270:129487. https://doi.org/10.1016/j.chemosphere.2020.129487
Dwijayanti U, Gunawan G, Widodo DS, Haris A, Suyati L, Lusiana RA (2020) Adsorpsi methylene blue (MB) menggunakan abu layang batubara teraktivasi larutan NaOH. Anal Environ Chem 5(1):1–14. https://doi.org/10.23960/aec.v5.i1.2020.p01-14
Fitriansyah A, Amir H, Elvinawati E (2021) Karakterisasi adsorben karbon aktif dari sabut pinang (Areca catechu) terhadap kapasitas adsorpsi zat warna indigosol blue 04-B. Alotrop 5(1):42–54
Habila MA, Al Othman ZA, El-Toni AM, Labis JP, Li X, Zhang F, Soylak M (2016) Mercaptobenzothiazole-functionalized magnetic carbon nanospheres of type Fe3O4@SiO2@C for the preconcentration of nickel, copper and lead prior to their determination by ICP-MS. Microchim Acta 183(8):2377–2384. https://doi.org/10.1007/s00604-016-1880-x
Irawati H, Aprilita NH, Sugiharto E (2018) Adsorpsi zat warna kristal violet menggunakan limbah kulit singkong (Manihot esculenta). Bimipa 25(1):17–31
Jiang F, Dinh DM, Hsieh Y. Lo. (2017) Adsorption and desorption of cationic malachite green dye on cellulose nanofibril aerogels. Carbohyd Polym 173:286–294. https://doi.org/10.1016/j.carbpol.2017.05.097
Khoshnamvand N, Jafari A, Kamarehie B, Mohammadi A, Faraji M (2019) Removal of malachite green dye from aqueous solutions using zeolitic imidazole framework-8. Environ Process 6(3):757–772. https://doi.org/10.1007/s40710-019-00384-9
Kurniawan A, Nizar M, Rijal M, Bagas R, Setyarsih W (2014) Studi pengaruh variasi suhu kalsinasi terhadap kekerasan bentuk morfologi, dan analisis porositas nanokomposit Cao/Sio2 untuk aplikasi bahan biomaterial. J Penelit Fis Dan Apl (JPFA) 4(2):22
Landin-Sandoval VJ, Mendoza-Castillo DI, Seliem MK, Mobarak M, Villanueva-Mejia F, Bonilla-Petriciolet A, Navarro-Santos P, Reynel-Ávila HE (2021) Physicochemical analysis of multilayer adsorption mechanism of anionic dyes on lignocellulosic biomasses via statistical physics and density functional theory. J Mol Liquids. https://doi.org/10.1016/j.molliq.2020.114511
Larosa C, Salerno M, de Lima JS, Merijs Meri R, da Silva MF, de Carvalho LB, Converti A (2018) Characterisation of bare and tannase-loaded calcium alginate beads by microscopic, thermogravimetric, FTIR and XRD analyses. Int J Biol Macromol 115:900–906. https://doi.org/10.1016/j.ijbiomac.2018.04.138
Leng L, Yuan X, Zeng G, Shao J, Chen X, Wu Z, Wang H, Peng X (2015) Surface characterization of rice husk bio-char produced by liquefaction and application for cationic dye (Malachite green) adsorption. Fuel 155:77–85. https://doi.org/10.1016/j.fuel.2015.04.019
Li N, Yue Q, Gao B, Xu X, Kan Y, Zhao P (2018) Magnetic graphene oxide functionalized by poly dimethyl diallyl ammonium chloride for efficient removal of Cr(VI). J Taiwan Inst Chem Eng 91:499–506. https://doi.org/10.1016/j.jtice.2018.05.028
Liang SX, Jia Z, Zhang WC, Wang WM, Zhang LC (2017) Rapid malachite green degradation using Fe73.5Si13.5B9Cu1Nb3 metallic glass for activation of persulfate under UV–Vis light. Mater Des 119:244–253
Mahreni M, Yuli R (2020) A review on Metal-Organic Framework (MOF): synthesis and solid catalyst applications. Proc Eng Sci Ser (ESS) 1(1):638–645
Manuja A, Kumar S, Dilbaghi N, Hanjana G, Chopra M, Kaur H, Kumar R, Manuja BK, Singh SK, Yadav SC (2014) Quinapyramine sulfate-loaded sodium alginat nanoparticles show enhanced trypanocidal activity. Nanomedicine 9:1625–1634
Marsen, Alimano dan Syafila Mindriany. (2014). Adsorbent size reduction to enlarge its pore diameter in effort to improve used cooking oil adsorption efficiency. Jurnal Teknik Lingkungan. Volume 20 Nomor 2, Oktober, pp. 173–182.
Neolaka YAB, Supriyanto G, Kusuma HS (2019) Synthesis and characterization of natural zeolite with ordered ion imprinted polymer structures (IIP@AFINZ) for selective Cr(VI) adsorption from aqueous solution. Moroccan J Chem 7(1):194–210
Neolaka YAB, Supriyanto G, Darmokoesoemo H, Kusuma HS (2018) Characterization, kinetic, and isotherm data for Cr(VI) removal from aqueous solution by Cr(VI)-imprinted poly(4-VP-co-MMA) supported on activated Indonesia (Ende-Flores) natural zeolite structure. Data Brief 17:969–979. https://doi.org/10.1016/j.dib.2018.01.076
Noreen S, Khalid U, Ibrahim SM, Javed T, Ghani A, Naz S, Iqbal M (2020) ZnO, MgO and FeO adsorption efficiencies for direct sky blue dye: equilibrium, kinetics and thermodynamics studies. J Mater Res Technol 9(3):5881–5893. https://doi.org/10.1016/j.jmrt.2020.03.115
Pan X, Zuo G, Su T, Cheng S, Gu Y, Qi X, Dong W (2019) Polycarboxylic magnetic polydopamine sub-microspheres for effective adsorption of malachite green. Colloids Surf A Physicochem Eng Aspects 560:106–113. https://doi.org/10.1016/j.colsurfa.2018.10.014
Patidar D, Goyal S (2021) Adsorptive removal of malachite green dye from aqueos solution using a non carbon adsorbent: equilibrium, kinetics and thermodynamics. J Adv Sci Res 1(2):19–23
Raval NP, Shah PU, Shah NK (2016) Nanoparticles loaded biopolymer as effective adsorbent for adsorptive removal of malachite green from aqueous solution. Water Conserv Sci Eng 1(1):69–81. https://doi.org/10.1007/s41101-016-0004-0
Rehman Shah HU, Ahmad K, Naseem HA, Parveen S, Ashfaq M, Rauf A, Aziz T (2021) Water stable graphene oxide metal-organic frameworks composite (ZIF-67@GO) for efficient removal of malachite green from water. Food Chem Toxicol 154(March):112312. https://doi.org/10.1016/j.fct.2021.112312
Sayğılı H, Güzel F (2018) Usability of activated carbon with well-developed mesoporous structure for the decontamination of malachite green from aquatic environments: kinetic, equilibrium and regeneration studies. J Porous Mater 25(2):477–488. https://doi.org/10.1007/s10934-017-0459-1
Shi Z, Xu C, Guan H, Li L, Fan L, Wang Y, Liu L, Meng Q, Zhang R (2018) Magnetic Metal Organic Frameworks (MOFs) composite for removal of lead and malachite green in wastewater. Colloids urf A: Physicochem Eng Aspects 539:382–390. https://doi.org/10.1016/j.colsurfa.2017.12.043
Tang J, Zhang YF, Liu Y, Li Y, Hu H (2020) Efficient ion-enhanced adsorption of congo red on polyacrolein from aqueous solution: experiments, characterization and mechanism studies. Sep Purif Technol 252(April):117445. https://doi.org/10.1016/j.seppur.2020.117445
Wu Y, Yang F, Liu X, Tan G, Xiao D (2018) Fabrication of N, P-codoped reduced graphene oxide and its application for organic dye removal. Appl Surf Sci 435:281–289. https://doi.org/10.1016/j.apsusc.2017.10.118
Zadvarzi SB, Khavarpour M, Vahdat SM, Baghbanian SM, Rad AS (2021) Synthesis of Fe3O4@chitosan@ZIF-8 towards removal of malachite green from aqueous solution: theoretical and experimental studies. Int J Biol Macromol 168:428–441. https://doi.org/10.1016/j.ijbiomac.2020.12.067
Zhang T, Jin X, Owens G, Chen Z (2021) Remediation of malachite green in wastewater by ZIF-8@Fe/Ni nanoparticles based on adsorption and reduction. J Colloid Interface Sci 594:398–408. https://doi.org/10.1016/j.jcis.2021.03.065
Zhao X, Zheng M, Gao X, Zhang J, Wang E, Gao Z (2021) The application of MOFs-based materials for antibacterials adsorption. Coord Chem Rev 440:213970. https://doi.org/10.1016/j.ccr.2021.213970
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahreni, M., Ramadhan, R.R., Pramadhana, M.F. et al. Synthesis of Metal Organic Framework (MOF) based Ca-Alginate for adsorption of malachite green dye. Polym. Bull. 79, 11301–11315 (2022). https://doi.org/10.1007/s00289-022-04086-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04086-5