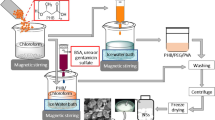
Abstract
The main objective of this study is to achieve a successful nanodrug delivery platform for Norfloxacin (NF) and Tenoxicame (TX) that allows sustained drug release in the body for treatment of microbial infections and pain revival. Poly(3-hydroxybutyrate) (PHB) are the most thoroughly studied forms of biopolymer polyesters family for biomedical applications due to their biocompatibility, biodegradability, sustained release of drugs and nontoxic. Malelic anhydride is used as compatibilizer between polymer and drugs. Nickel oxide (NiO), ferromagnetic material, has been applied in the medical field and platforms for drug delivery system, so spherical crystalline NiO nanoparticles were dispersed in the polymer matrix. PHB grafted maleic anhydride/NiO nanocomposites (PHB-g-MA-NiO) was designed and used as a dual carrier for NF and TX. The crystallite size and type of interaction between functional groups were characterized by XRD and FTIR techniques. The thermal behaviour of PHB-g-MA-NiO nanocomposites was characterized by TGA and compared with PHB, PHB-g-MA. Firstly, PHB-g-MA-NiO was used with different contents of NiO as a carrier for TX. The drug release mechanism was studied using Zero-order, First-order, Higuchi, Hixon–Crowell and Korsmeyer–Peppas equations at different pH (2, 7.4). The carrier with the highest loading % of TX was loaded by NF and a mixture of NF/TX drugs. The PHB-g-MA-5%NiO nanocomposite loaded NF/TX showed potential activity against Staphylococcus aureus (Gram-positive), Klebsiella pneumonia (Gram-negative) and Bacillus cereus (Gram-positive). NF/TX/PHB-g-MA-5%NiO nanocomposite was recorded to release 100% and 14% of NF and TX, respectively after 21 days at pH = 2. The % of cumulative release of NF and TX after 504 h at pH 2 illustrated a high potential of PHB-g-MA-5%NiO nanocomposite loaded by NF/TX for usage as a drug carrier.
Graphical abstract










Similar content being viewed by others
References
Huang S, Fu X (2010) Naturally derived materials-based cell and drug delivery systems in skin regeneration. J Control Release 142(2):149–159
Abdelwahab M, Salahuddin N, Gaber M, Mousa M (2018) Poly(3-hydroxybutyrate)/polyethylene glycol-NiO nanocomposite for NOR delivery: antibacterial activity and cytotoxic effect against cancer cell lines. Int J Biol Macromol 114:717–727
Abdelwahab MA, El-Barbary AA, El-Said KS, Betiha M, Elkholy HM, Chiellini E, El-Magd MA (2019) Functionalization of poly(3-hydroxybutyrate) with different thiol compounds inhibits MDM2–p53 interactions in MCF7 cells. J Appl Polym Sci 136(2):46924
Abdelwahab MA, El-Barbary AA, El-Said KS, El Naggar SA, ElKholy HM (2019) Evaluation of antibacterial and anticancer properties of poly(3-hydroxybutyrate) functionalized with different amino compounds. Int J Biol Macromol 122:793–805
Salahuddin N, Abdelwahab M, Gaber M, Elneanaey S (2020) Synthesis and design of norfloxacin drug delivery system based on PLA/TiO2 nanocomposites: antibacterial and antitumor activities. Mater Sci Eng C 108:110337
Salahuddin N, Gaber M, Mousa M, Abdelwahab MA (2020) Poly(3-hydroxybutyrate)/poly(amine)-coated nickel oxide nanoparticles for norfloxacin delivery: antibacterial and cytotoxicity efficiency. RSC Adv 10(56):34046–34058
Tesema Y, Raghavan D, Stubbs J III (2004) Bone cell viability on collagen immobilized poly (3-hydroxybutrate-co-3-hydroxyvalerate) membrane: effect of surface chemistry. J Appl Polym Sci 93(5):2445–2453
Sodian R, Hoerstrup SP, Sperling JS, Martin DP, Daebritz S, Mayer JE Jr, Vacanti JP (2000) Evaluation of biodegradable, three-dimensional matrices for tissue engineering of heart valves. ASAIO J 46(1):107–110
Li H, Chang J (2005) Preparation, characterization and in vitro release of gentamicin from PHBV/wollastonite composite microspheres. J Control Release 107(3):463–473
Akiyama M, Tsuge T, Doi Y (2003) Environmental life cycle comparison of polyhydroxyalkanoates produced from renewable carbon resources by bacterial fermentation. Polym Degrad Stab 80(1):183–194
Hassan MA, Yee L-N, Yee PL, Ariffin H, Raha AR, Shirai Y, Sudesh K (2013) Sustainable production of polyhydroxyalkanoates from renewable oil-palm biomass. Biomass Bioenergy 50:1–9
Vert M, Li S, Spenlehauer G, Guérin P (1992) Bioresorbability and biocompatibility of aliphatic polyesters. J Mater Sci Mater Med 3(6):432–446
Pamula E, Dobrzynski P, Szot B, Kretek M, Krawciow J, Plytycz B, Chadzinska M (2008) Cytocompatibility of aliphatic polyesters—in vitro study on fibroblasts and macrophages. J Biomed Mater Res Part A 87(2):524–535
Romero AI, Bermudez JM, Villegas M, Ashur MFD, Parentis ML, Gonzo EE (2016) Modeling of progesterone release from poly (3-hydroxybutyrate)(PHB) membranes. AAPS PharmSciTech 17(4):898–906
Shah M, Naseer MI, Choi MH, Kim MO, Yoon SC (2010) Amphiphilic PHA–mPEG copolymeric nanocontainers for drug delivery: preparation, characterization and in vitro evaluation. Int J Pharm 400(1–2):165–175
Avella M, Bogoeva-Gaceva G, Buzoarovska A, Emanuela Errico M, Gentile G, Grozdanov A (2007) Poly (3-hydroxybutyrate-co-3-hydroxyvalerate)-based biocomposites reinforced with kenaf fibers. J Appl Polym Sci 104(5):3192–3200
Modesti M, Lorenzetti A, Bon D, Besco S (2005) Effect of processing conditions on morphology and mechanical properties of compatibilized polypropylene nanocomposites. Polymer 46(23):10237–10245
Vardhan H, Mittal P, Adena SKR, Mishra B (2017) Long-circulating polyhydroxybutyrate-co-hydroxyvalerate nanoparticles for tumor targeted docetaxel delivery: formulation, optimization and in vitro characterization. Eur J Pharm Sci 99:85–94
Kwon H-S, Jung S-G, Kim H-Y, Parker SA, Batt CA, Kim Y-R (2014) A multi-functional polyhydroxybutyrate nanoparticle for theranostic applications. J Mater Chem B 2(25):3965–3971
Bucak S, Yavuztürk B, Sezer AD Magnetic nanoparticles: synthesis, surface modifications and application in drug delivery, Recent Advances in Novel Drug Carrier Systems, InTech2012
Berkovsky B (1993) Magnetic fluids. Eng Appl 1993:171–213
Häfeli U, Schütt W, Teller J, Zborowski M (2013) Scientific and clinical applications of magnetic carriers. Springer, Berlin
Yoon T-J, Lee W, Oh Y-S, Lee J-K (2003) Magnetic nanoparticles as a catalyst vehicle for simple and easy recycling. New J Chem 27(2):227–229
Oliveira-Silva R, Pereira RA, Silva FM, Gaspar VM, Ibarra A, Millán Á, Sousa FL, Mano JF, Silva NJ (2019) Temperature-responsive nanomagnetic logic gates for cellular hyperthermia. Mater Horiz 6(3):524–530
Jose J, Kumar R, Harilal S, Mathew GE, Prabhu A, Uddin MS, Aleya L, Kim H, Mathew B (2020) Magnetic nanoparticles for hyperthermia in cancer treatment: an emerging tool. Environ Sci Pollut Res 27(16):19214–19225
Shen L, Chu D (1996) Type II DNA topoisomerases as antibacterial targets. Curr Pharm Des 2(2):195–208
Marians KJ, Hiasa H (1997) Mechanism of quinolone action a drug-induced structural perturbation of the DNA precedes strand cleavage by topoisomerase IV. J Biol Chem 272(14):9401–9409
Cozzarelli NR (1980) DNA gyrase and the supercoiling of DNA. Science 207(4434):953–960
Frimodt-Møller P, Jensen K, Madsen P (1983) Antibacterial activity of norfloxacin in the gastrointestinal tracts of rats. Antimicrob Agents Chemother 24(4):560–563
Halkin H (1988) Adverse effects of the fluoroquinolones. Rev Infect Diseases 10(Supplement 1):S258–S261
Norrby S (1991) Side-effects of quinolones: comparisons between quinolones and other antibiotics. Eur J Clin Microbiol Infect Dis 10(4):378–383
Kim GK (2010) The risk of fluoroquinolone-induced tendinopathy and tendon rupture: what does the clinician need to know? J Clin Aesthet Dermatol 3(4):49
Moseley RH (2013) Hepatotoxicity of antimicrobials and antifungal agents, Drug-Induced Liver Disease, 3rd edn. Elsevier, New York, pp 463–481
Evstigneev M, Rybakova K, Davies D (2007) Formation of complexes of antimicrobial agent norfloxacin with antitumor antibiotics of anthracycline series. Russ J Phys Chem A 81(5):802–807
Sobczak M, Nurzyńska K, Kolodziejski W (2010) Seeking polymeric prodrugs of norfloxacin. Part 2. Synthesis and structural analysis of polyurethane conjugates. Molecules 15(2):842–856
Martins DA, Gouvea LR, Muniz GSV, Louro SR, Batista DDGJ, Soeiro MDNC, Teixeira LR (2016) Norfloxacin and N-Donor mixed-ligand Copper (II) complexes: synthesis, albumin interaction, and anti-Trypanosoma cruzi activity. Bioinorgan Chem Appl 2016:5027404–5027415.
Wiseman E, Lombardino J (1981) Oxicams—a novel family of non-steroidal anti-inflammatory drugs. Eur J Rheumatol Inflamm 4(3):280
Laine L (1996) Nonsteroidal anti-inflammatory drug gastropathy. Gastrointest Endosc Clin N Am 6(3):489–504
Wallace JL (1997) Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology 112(3):1000–1016
Mizuno H, Sakamoto C, Matsuda K, Wada K, Uchida T, Noguchi H, Akamatsu T, Kasuga M (1997) Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology 112(2):387–397
Vagare RS (2015) Microbial triggered colon targeted compression coated tablets of tenoxicam: formulation and evaluation. J Drug Deliv Therap 5(1):75–81
Muenprasat D, Suttireungwong S, Tongpin C (2010) Functionalization of poly (lactic acid) with maleic anhydride for biomedical application. J Met Mater Miner 20(3):189–192
Wu C-S (2003) Physical properties and biodegradability of maleated-polycaprolactone/starch composite. Polym Degrad Stab 80(1):127–134
Oromiehie A, Ebadi-Dehaghani H, Mirbagheri S (2014) Chemical modification of polypropylene by maleic anhydride: melt grafting, characterization and mechanism. Int J Chem Eng Appl 5(2):117
Thota S, Kumar J (2007) Sol–gel synthesis and anomalous magnetic behaviour of NiO nanoparticles. J Phys Chem Solids 68(10):1951–1964
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA (1983) Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 15(1):25–35
Botana A, Mollo M, Eisenberg P, Sanchez RMT (2010) Effect of modified montmorillonite on biodegradable PHB nanocomposites. Appl Clay Sci 47(3):263–270
Zhijiang C, Chengwei H, Guang Y (2011) Crystallization behavior, thermal property and biodegradation of poly (3-hydroxybutyrate)/poly (ethylene glycol) grafting copolymer. Polym Degrad Stab 96(9):1602–1609
Mottin AC, Ayres E, Oréfice RL, Câmara JJD (2016) What changes in poly (3-hydroxybutyrate)(PHB) when processed as electrospun nanofibers or thermo-compression molded film? Mater Res 19(1):57–66
Uzun G, Aydemir D (2017) Biocomposites from polyhydroxybutyrate and bio-fillers by solvent casting method. Bull Mater Sci 40(2):383–393
Galego N, Rozsa C, Sánchez R, Fung J, Vázquez AA, Santo Tomás J (2000) Characterization and application of poly (β-hydroxyalkanoates) family as composite biomaterials. Polym Test 19(5):485–492
Hofmeister H (2005) Tan GL and Dubiel M. J Mater Res 2005:20
Salahuddin N, Gaber M, Mousa M, Abdelwahab MA (2020) Poly (3-hydroxybutyrate)/poly (amine)-coated nickel oxide nanoparticles for norfloxacin delivery: antibacterial and cytotoxicity efficiency. RSC Adv 10(56):34046–34058
Arslan H (2007) Formulation study for enteric microspheres of tenoxicam using cellulose acetate phthalate part-II: modulation of ulcerogenic effect¦. Asian J Chem 19(7):5718
Elkomy MH, El Menshawe SF, Eid HM, Ali AM (2017) Development of a nanogel formulation for transdermal delivery of tenoxicam: a pharmacokinetic–pharmacodynamic modeling approach for quantitative prediction of skin absorption. Drug Dev Ind Pharm 43(4):531–544
Guguloth M, Bomma R, Veerabrahma K (2011) Development of sustained release floating drug delivery system for norfloxacin: in vitro and in vivo evaluation. PDA J Pharm Sci Technol 65(3):198–206
Kılıçay E, Demirbilek M, Türk M, Güven E, Hazer B, Denkbas EB (2011) Preparation and characterization of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate)(PHBHHX) based nanoparticles for targeted cancer therapy. Eur J Pharm Sci 44(3):310–320
Calamak S, Shahbazi R, Eroglu I, Gultekinoglu M, Ulubayram K (2017) An overview of nanofiber-based antibacterial drug design. Expert Opin Drug Discov 12(4):391–406
Shaikh HK, Kshirsagar R, Patil S (2015) Mathematical models for drug release characterization: a review. Wjpps 4:324–338
Abdel-Aziz AA-M, El-Azab AS, Alanazi AM, Asiri YA, Al-Suwaidan IA, Maarouf AR, Ayyad RR, Shawer TZ (2016) Synthesis and potential antitumor activity of 7-(4-substituted piperazin-1-yl)-4-oxoquinolines based on ciprofloxacin and norfloxacin scaffolds: in silico studies. J Enzyme Inhib Med Chem 31(5):796–809
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salahuddin, N., Gaber, M., Mousa, M. et al. Dual drug delivery system based on biodegradable modified poly(3-hydroxybutyrate)-NiO nanocomposite and sequential release of drugs. Polym. Bull. 79, 10969–10990 (2022). https://doi.org/10.1007/s00289-021-04029-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-04029-6