Abstract
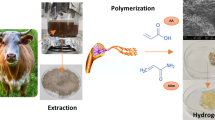
Human hair is a slow-degrading nanocomposite biological fiber. In the present work, the surface of human hair has been modified via grafting of poly(methyl methacrylate). The grafting has been done via free radical polymerization using graft from approach. The percent grafting calculated from thermo-gravimetric analysis data was in good agreement with the percent grafting calculated from gravimetric method. The scanning electron microscopy images showed that the hair surface got completely covered when the weight of methyl methacrylate was twice that of human hair in the feed. The ultimate tensile strength and modulus were found to be 1099 MPa and 20 GPa, respectively, when hair was grafted with feed ratio of 2:1 for methyl methacrylate and hair, as compared to 795 MPa and 16 GPa, respectively, for virgin human hair. An improvement in chemical stability was also observed on grafting, under both basic and acidic conditions. The effect of grafting on swelling and adsorption properties has also been studied. For a lower contact time, the removal efficiency was found to be more for anionic dye, methyl orange as compared to cationic dye, methylene blue, but as the contact time increased, the removal efficiency of grafted copolymers for methylene blue increased significantly. The effect of contact time, pH, adsorbent dosage, initial dye concentration on absorption and desorption studies has also been done. The adsorption behavior was studied using isotherm models Langmuir, Freundlich and Temkin model, and adsorption kinetics were investigated using pseudo-first-order and pseudo-second-order model.




























Similar content being viewed by others
Abbreviations
- AIBN:
-
Azobisisobutyronitrile
- BIS:
-
N, N′-methylenebisacrylamide
- DSC:
-
Differential scanning calorimeter
- DMA:
-
Dynamic mechanical analyzer
- FT-IR:
-
Fourier transform infrared spectroscopy
- HH:
-
Human hair
- HHact :
-
Activated human hair
- MMA:
-
Methyl methacrylate
- MB:
-
Methylene blue
- MO:
-
Methyl orange
- PMMA:
-
Poly(methyl methacrylate)
- SEM:
-
Surface electron microscopy
- TGA:
-
Thermogravimetric analysis
- THF:
-
Tetrahydrofuran
- UTS:
-
Ultimate tensile strength
- b:
-
Langmuir constant related with energy of adsorption
- b T :
-
Heat of adsorption
- C e :
-
Concentration at equilibrium
- C o :
-
Initial concentration
- C f :
-
Final concentration
- K :
-
Rate constant
- K f :
-
Freundlich isotherm constants
- K T :
-
Equilibrium binding constant
- M :
-
Weight of adsorbent
- n :
-
Adsorption intensity
- Q e :
-
Desorption capacity at equilibrium
- Q t :
-
Sorption capacities at time t
- q e :
-
Adsorption capacity at equilibrium
- q m :
-
Langmuir constant
- q t :
-
Adsorption capacity at time t
- R :
-
Universal gas constant
- R 2 :
-
Correlation coefficient
- T :
-
Absolute temperature
- V :
-
Volume of dye solution
- W i :
-
Initial weight of polymer
- W f :
-
Final weight of polymer
- η :
-
Removal efficiency
References
Xueliang X (2020) Animal Fibers. In: Hu J (ed) Handbook of fibrous materials, 1st edn. Wiley-VCH, pp 35–74. https://doi.org/10.1002/9783527342587.ch2
Gupta A (2014) Human hair “waste” and its utilization: gaps and possibilities. J Waste Manag. https://doi.org/10.1155/2014/498018
Martínez-Hernández AL, Velasco-Santos C, de-Icaza M, Castaño VM (2007) Dynamical-mechanical and thermal analysis of polymeric composites reinforced with keratin biofibers from chicken feathers. Compos Part B Eng 38:405–410. https://doi.org/10.1016/j.compositesb.2006.06.013
Verma A, Singh VK, Verma SK, Sharma A (2016) Human hair: a biodegradable composite fiber–a review. Int J Waste Resour. https://doi.org/10.4172/2252-5211.1000206
Kaplin IJ, Schwan A, Zahn H (1982) Effects of cosmetic treatments on the ultrastructure of hair. Cosmet Toilet 97:22–26
Wagner RDCC, Joekes I (2005) Hair protein removal by sodium dodecyl Sulfate. Colloids Surf B Biointerf 41:7–14. https://doi.org/10.1016/j.colsurfb.2004.10.023
Prasong S, Wasan T (2011) Preparation and characterization of hair keratin/gelatin blend films. Pakistan J Biol Sci 14:351–356. https://doi.org/10.3923/pjbs.2011.351.356
Bertini F, Canetti M, Patrucco A, Zoccola M (2013) Wool keratin-polypropylene composites: properties and thermal degradation. Polym Degrad Stab 98:980–987. https://doi.org/10.1016/j.polymdegradstab.2013.02.011
Baghdadli N, Luengo GS (2008) A closer look at the complex hydrophilic/hydrophobic interactions forces at the human hair surface. J Phys Conf Ser 100:3–7. https://doi.org/10.1088/1742-6596/100/5/052034
Velasco MVR, de Sá Dias TC, de Freitas AZ, Junior NDV, de Oliveira Pinto CAS, Kaniko TM, Baby AR (2009) Hair fiber characteristics and methods to evaluate hair physical and mechanical properties. Braz J Pharm Sci 45:153–162. https://doi.org/10.1590/S1984-82502009000100019
Bhushan B, Chen N (2006) AFM studies of environmental effects on nanomechanical properties and cellular structure of human hair. Ultramicroscopy 106:755–764. https://doi.org/10.1016/j.ultramic.2005.12.010
Robbins CR, Crawford RJ (1991) Cuticle damage and the tensile properties of human hair. J Soc Cosmet Chem 42:59–67
Feughelman M (1997) Morphology and properties of hair. In: Johnson DH (ed) Hair and hair care. Marcel Dekker, New York, pp 1–32
Wang N, Barfoot R, Butler MF, Durkan C (2018) Effect of surface treatments on the nanomechanical properties of human hair. ACS Biomater Sci Eng 4:3063–3071. https://doi.org/10.1021/acsbiomaterials.8b00687
Grams YY, Alaruikka S, Lashley L, Caussin J, Whitehead L (2003) Permeant lipophilicity and vehicle composition influence accumulation of dyes in hair follicles of human skin. Eur J Pharm Sci 18:329–336. https://doi.org/10.1016/S0928-0987(03)00035-6
McFadden JP, White IR, Frosch PJ, Sosted H, Johansen JD, Menne T (2007) Allergy to hair dye–its incidence is rising, as more and younger people dye their hair. Br Med J 334:220. https://doi.org/10.1136/bmj.39042.643206.BE
Freddi G, Tsukada M, Shiozaki H (1999) Chemical modification of wool fibers with acid anhydrides. J Appl Polym Sci 71:1573–1579. https://doi.org/10.1002/(sici)1097-4628(19990307)71:10%3c1573::aid-app5%3e3.0.co;2-a
Abdel-Fattah S, Geczy I (1974) Graft polymerization of methyl methacrylate onto wool initiated by hydrogen peroxide—sodium thiosulphate redox system. Polym J 6:542–548. https://doi.org/10.1295/polymj.6.542
Asquith RS, Leon NH (1977) Chemical reactions of keratin fibres. Springer, pp 193–265. https://doi.org/10.1007/978-1-4613-4109-3_5
Zhang H, Carrillo-Navarrete F, López-Mesas M, Palet C (2020) Use of chemically treated human hair wastes for the removal of heavy METAL ions from water. Water 12:1–17. https://doi.org/10.3390/W12051263
Malinauskyte E, Cornwell PA, Reay L, Shaw N, Petkov J (2020) Effect of equilibrium pH on the structure and properties of bleach-damaged human hair fibers. Biopolymers. https://doi.org/10.1002/bip.23401
Bolduc C, Shapiro J (2001) Hair care products: waving, straightening, conditioning, and coloring. Clin Dermatol 19:431–436. https://doi.org/10.1016/S0738-081X(01)00201-2
Sharma G, Naushad M, Pathania D, Mittal A, El-Desoky GE (2014) Modification of hibiscus cannabinus fiber by graft copolymerization: application for dye removal. Desalin Water Treat 54:3114–3121. https://doi.org/10.1080/19443994.2014.904822
Shi Z, Reddy N, Hou X, Yang Y (2014) Development and characterization of thermoplastics from corn distillers grains grafted with various methacrylates. Ind Eng Chem Res 53:13963–13970. https://doi.org/10.1021/ie501987n
Candido ICM, Pires ICB, de Oliveira HP (2021) Natural and synthetic fiber-based adsorbents for water remediation. Clean Soil Air Water 49:1–11. https://doi.org/10.1002/clen.202000189
Chowdhury S, Saha PD (2012) Biosorption of methylene blue from aqueous solutions by a waste biomaterial: hen feathers. Appl Water Sci 2:209–219. https://doi.org/10.1007/s13201-012-0039-0
Gao P, Li K, Liu Z, Liu B, Ma C, Xue G, Zhou M (2014) Feather keratin deposits as biosorbent for the removal of methylene blue from aqueous solution: equilibrium, kinetics, and thermodynamics studies. Water Air Soil Pollut. https://doi.org/10.1007/s11270-014-1946-5
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80. https://doi.org/10.1016/j.jhazmat.2009.12.047
Darmokoesoemo H, Magdhalena PTWLC, Kusuma HS (2016) Telescope snail (Telescopium Sp) and mangrove crab (Scylla Sp) as adsorbent for the removal of Pb2+ from aqueous solutions. Rasayan J Chem 9:680–685
Darmokoesoemo H, Setianingsih FR, Putranto TWLC, Kusuma HS (2016) Horn snail (Telescopium Sp) and mud crab (Scylla Sp) shells powder as low cost adsorbents for removal of Cu2+ from synthetic wastewater. Rasayan J Chem 9:550–555
Thakur VK, Thakur MK, Gupta RK (2014) Graft copolymers of natural fibers for green composites. Carbohydr Polym 104:87–93. https://doi.org/10.1016/j.carbpol.2014.01.016
Kalia S, Kaith BS, Kaur I (2009) Pretreatments of natural fibers and their application as reinforcing material in polymer composites—a review. Polym Eng Sci 49:1253–1272. https://doi.org/10.1002/pen
Morinaga H, Ochiai B, Mori H, Endo T (2006) Anionic grafting polymerization of propylene sulfide onto human hair in water. J Polym Sci Part A Polym Chem 44:3778–3786. https://doi.org/10.1002/pola.21478
Lee S, Zürcher S, Dorcier A, Luengo GS, Spencer ND (2009) Adsorption and lubricating properties of Poly(l-lysine)-graft-Poly(ethylene glycol) on human-hair surfaces. ACS Appl Mater Interf 1:1938–1945. https://doi.org/10.1021/am900337z
Wang L, Cavaco-Paulo A, Xu B, Martins M (2019) Polymeric hydrogel coating for modulating the shape of keratin fiber. Front Chem. https://doi.org/10.3389/fchem.2019.00749
Robbins CR, Robbins CR (1988) Bleaching human hair. Chem Phys Behav Hum Hair. https://doi.org/10.1007/978-1-4757-2009-9_4
Xu H, Song K, Mu B, Yang Y (2017) Green and sustainable technology for high-efficiency and low-damage manipulation of densely crosslinked proteins. ACS Omega 2:1760–1768. https://doi.org/10.1021/acsomega.7b00154
Grassi L, Cabrele C (2019) Susceptibility of protein therapeutics to spontaneous chemical modifications by oxidation, cyclization, and elimination reactions. Amino Acids 51:1409–1431. https://doi.org/10.1007/s00726-019-02787-2
Davies MJ (2016) Protein oxidation and peroxidation. Biochem J 473:805–825. https://doi.org/10.1042/BJ20151227
Fairbanks BD, Singh SP, Bowman CN, Anseth KS (2011) Photodegradable, photoadaptable hydrogels via radical-mediated disulfide fragmentation reaction. Macromolecules 44:2444–2450. https://doi.org/10.1021/ma200202w
Ji Y, Yang X, Ji Z, Zhu L, Ma N, Chen D, Jia X, Tang J, Cao Y (2020) DFT-calculated IR spectrum amide I, II, and III band contributions of N-Methylacetamide fine components. ACS Omega 5:8572–8578. https://doi.org/10.1021/acsomega.9b04421
Hopkins J, Brenner L, Tumosa CS (1991) Variation of the amide I and amide II peak absorbance ratio in human hair as measured by fourier transform infrared spectroscopy. Forensic Sci Int 50:61–65. https://doi.org/10.1016/0379-0738(91)90134-5
Parker FS (1971) Amides and amines. Appl Infrared Spectrosc Biochem Biol Med 1:165–172. https://doi.org/10.1007/978-1-4684-1872-9_8
Mujeeb MA, Zafar MKM (2017) FTIR spectroscopic analysis on human hair. Int J Innov Res Sci Eng Technol 6:9327–9332. https://doi.org/10.15680/IJIRSET.2017.0605195
Terashima M, Yoshimura K, Imai T, Hozan D, Shirai K (2000) Properties of protein extracted as S-sulfonate derivative from irradiated mink hair. Anim Sci J 71:76–82
Deoghare C, Nadkarni VS, Behera RN, Chauhan R (2019) Copolymers with pendant N-arylimide groups via atom transfer radical polymerization: synthesis, characterization and kinetic study. Polym Sci Ser B 61:170–179. https://doi.org/10.1134/S1560090419020015
Xiao X, Hu J (2016) Animal hairs as water-stimulated shape memory materials: mechanism and structural networks in molecular assemblies. Sci Rep 6:1–12. https://doi.org/10.1038/srep26393
Embi Bs AA (2018) Absence of H2O2 breakdown in human hair medulla implications IN follicular melanogenesis. Int J Res Granthaalayah 6:72–78. https://doi.org/10.29121/granthaalayah.v6.i9.2018.1209
Feughelman M (1959) The change in stress on wetting and drying wool fibers. Text Res J 29:967–970. https://doi.org/10.1177/004051755902901205
Wortmann FJ, Zahn H (1994) The stress/strain curve of α-keratin fibres and the structure of the intermediate filament. Text Res J 64:737–743
Chapman BM (1969) A mechanical model for wool and other keratin fibers. Text Res J 39:1102–1109. https://doi.org/10.1177/004051756903901204
Feughelman M (1979) A note on the role of the microfibrils in the mechanical properties of α-keratins. J Macromol Sci Part B Phy 16:155–162. https://doi.org/10.1080/00222347908212288
Deepmala K, Jain N, Singh VK, Chauhan S (2018) Fabrication and characterization of chitosan coated human hair reinforced phytagel modified soy protein-based green composite. J Mech Behav Mater 27:1–8. https://doi.org/10.1515/jmbm-2018-0007
Robbins CR (2012) Chemical and physical behavior of human hair, 5th edn. Springer, Berlin
Maddar FM, Perry D, Brooks R, Page A, Unwin PR (2019) Nanoscale surface charge visualization of human hair. Anal Chem 91:4632–4639. https://doi.org/10.1021/acs.analchem.8b05977
Popescu C, Höcker H (2007) Hair—the most sophisticated biological composite material. Chem Soc Rev 36:1282–1291. https://doi.org/10.1039/b604537p
Giraldo S, Robles I, Godínez LA, Acelas N, Florez E (2021) Experimental and theoretical insights on methylene blue removal from wastewater USING an adsorbent obtained from the residues of the orange industry. Molecules 26:4555. https://doi.org/10.3390/molecules26154555
Chandrashekara MN, Ranganathaiah C (2010) Chemical and photochemical degradation of human hair: a free-volume microprobe study. J Photochem Photobiol B Biol 101:286–294. https://doi.org/10.1016/j.jphotobiol.2010.07.014
Hu Y, Quan C, Guo M, Ye X, Wu Z (2017) Competitive adsorption of methyl orange and ethyl orange by AB-8 resin. Emerg Mater Res 6:369–377. https://doi.org/10.1680/jemmr.15.00082
Chen H, Zhao J, Wu J, Dai G (2011) Isotherm, thermodynamic, kinetics and adsorption mechanism studies of methyl orange by SURFACTANT modified silkworm exuviae. J Hazard Mater 192:246–254. https://doi.org/10.1016/j.jhazmat.2011.05.014
Le GTT, Chanlek N, Manyam J, Opaprakasit P, Grisdanurak N, Sreearunothai P (2019) Insight into the ultrasonication of graphene oxide with strong changes in its properties and performance for adsorption applications. Chem Eng J 373:1212–1222. https://doi.org/10.1016/j.cej.2019.05.108
Al-Ghouti MA, Al-Absi RS (2020) Mechanistic understanding of the adsorption and thermodynamic aspects of cationic methylene blue dye onto cellulosic olive stones biomass from wastewater. Sci Rep 10:15928. https://doi.org/10.1038/s41598-020-72996-3
Franca AS, Oliveira LS, Ferreira ME (2009) Kinetics and equilibrium studies of methylene blue adsorption by spent coffee grounds. Desalination 249:267–272. https://doi.org/10.1016/j.desal.2008.11.017
Aluigi A, Rombaldoni F, Tonetti C, Jannoke L (2014) Study of methylene blue adsorption on keratin nanofibrous membranes. J Hazard Mater 268:156–165. https://doi.org/10.1016/j.jhazmat.2014.01.012
Parreira HC (1980) On the isoelectric point of human hair. J Colloid Interf Sci 75:212–217. https://doi.org/10.1016/0021-9797(80)90363-X
Ayawei N, Ebelegi AN, Wankasi D (2017) Modelling and interpretation of adsorption isotherms. J Chem. https://doi.org/10.1155/2017/3039817
Song G, Zhu X, Chen R, Lio Q, Ding Y-D, Chen L (2016) An investigation of CO2 adsorption kinetics on porous magnesium oxide. Chem Eng J 283:175–183. https://doi.org/10.1016/j.cej.2015.07.055
Azizian S (2004) Kinetic models of sorption: a theoretical analysis. J Colloid Interf Sci 276:47–52. https://doi.org/10.1016/j.jcis.2004.03.048
Sahoo TR, Prelot B (2020) Adsorption processes for the removal of contaminants from wastewater. Elsevier Inc
Mercado-Borrayo BM, Schouwenaars R, Litter MI, Montoya-Bautista CV, Ramirez-Zamora RMS (2014) Metallurgical slag as an efficient and economical adsorbent of arsenic. Elsevier Inc
El Sikaily A, Khaled A, El Nemr A, Abdelwahab O (2006) Removal of methylene blue from aqueous solution by marine green alga ulva lactuca. Chem Ecol 22:149–157. https://doi.org/10.1080/02757540600579607
Bhattacharya KG, Sharma A (2005) Kinetics and thermodynamics of methylene blue adsorption on neem (Azadirachta Indica) leaf powder. Dye Pigment 65:51–59. https://doi.org/10.1016/j.dyepig.2004.06.016
Ahmad Zaini MA, Sudi RM (2018) Valorization of human hair as methylene blue dye adsorbents. Green Process Synth 7:344–352. https://doi.org/10.1515/gps-2017-0021
Salisu A, Sanagi MM, Karim KJA et al (2015) Adsorption of methylene blue on alginate-grafted-poly (methyl methacrylate). J Teknol 13:19–25
Pathania D, Sharma S, Singh P (2017) Removal of methylene blue by adsorption onto activated carbon developed from Ficus Carica bast. Arab J Chem 10:S1445–S1451. https://doi.org/10.1016/j.arabjc.2013.04.021
Basava Rao VV, Ram Mohan Rao S (2006) Adsorption studies on treatment of textile dyeing industrial effluent by flyash. Chem Eng J 116:77–84. https://doi.org/10.1016/j.cej.2005.09.029
El Qada EN, Allen SJ, Walker GM (2008) Adsorption of basic dyes from aqueous solution onto activated carbons. Chem Eng J 135:174–184. https://doi.org/10.1016/j.cej.2007.02.023
Fernandes AN, Almeida CAP, Menezes CTB et al (2007) Removal of methylene blue from aqueous solution by peat. J Hazard Mater 144:412–419. https://doi.org/10.1016/j.jhazmat.2006.10.053
Acknowledgements
RC and HS are thankful to the Central Sophisticated Instrumentation Facility (CSIF) of BITS Pilani, K. K. Birla Goa campus, for providing the FE-SEM and Raman facility, and G. S. Mandal’s M-CAMRT, Aurangabad, for DSC and FT-IR. The support from BITS Pilani, K. K. Birla Goa Campus, in terms of fellowship for HS is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
HS carried out the experimental work and was involved in data interpretation and manuscript writing. SW provided the facilities for testing mechanical properties and was involved in data interpretation and manuscript writing. RC has conceived the idea, provided raw materials and was involved in data interpretation and manuscript writing. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no personal, financial or organizational conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Srivastava, H., Waigaonkar, S. & Chauhan, R. Surface modification of human hair by grafting poly(methyl methacrylate). Polym. Bull. 79, 11013–11050 (2022). https://doi.org/10.1007/s00289-021-03990-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03990-6