Abstract
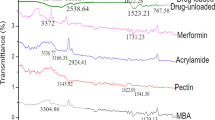
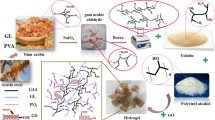
In the present study, main complication associated with duodenal ulcer is bleeding that was due to frequent use of NSAIDs, which was treated by ranitidine HCL (RNT). For the targeted release of RNT, pH-sensitive gelatin/acrylamide networks were synthesized by free radical polymerization, and the effect of different concentrations of initiator, monomer, polymer, and cross-linker on swelling was observed. Average molecular weight (Mc), volume fraction of the polymer V2, solvent interaction parameter, and cross-linked density were calculated. FTIR and SEM studies were performed for the interaction and morphological studies of networks. Dissolution efficiency (DE) and percentage release of RNT were analyzed in acidic media as well as in alkaline phosphate buffer pH 7.5. 80% swelling in phosphate buffer of pH 7.5 in 12 h showed inverse behavior with the concentration of cross-linker and direct relation with the gelatin. Akaike information criteria (AIC) were used for the selection of one best model. Formulation AAm1Ge1 at pH 7.5 has the best fitting curve with maximum regression coefficient 0.94 and minimum 66 AIC values. 80% RNT release with non-Fickian behavior and was observed in all prepared networks. Proposed networks formulations can be used for the treatment of NSAIDs induced duodenal ulcer.






Similar content being viewed by others
References
Minhas MU et al (2020) Functionalized pectin hydrogels by cross-linking with monomer: synthesis, characterization, drug release and pectinase degradation studies. Polym Bull 77(1):339–356
Nguyen MK, Lee DS (2010) Injectable biodegradable hydrogels. Macromol Biosci 10(6):563–579
Chuysinuan P et al (2019) Development of gelatin hydrogel pads incorporated with Eupatorium adenophorum essential oil as antibacterial wound dressing. Polym Bull 76(2):701–724
Chern JM, Lee WF, Hsieh MY (2004) Preparation and swelling characterization of poly (n-isopropylacrylamide)-based porous hydrogels. J Appl Polym Sci 92(6):3651–3658
Karadağ E et al (2020) Swelling equilibria of novel propenamide/2-acrylamide o-2-methyl-1-propanesulfonic acid/guar gum/clinoptilolite biohybrid hydrogels and application as a sorbent for BV1 removal. Polym Bull 78(7):3625–3649
Satish C, Satish K, Shivakumar H (2006) Hydrogels as controlled drug delivery systems: synthesis, crosslinking, water and drug transport mechanism. Indian J Pharm Sci 68(2):133–140
Bajpai AK et al (2008) Responsive polymers in controlled drug delivery. Prog Polym Sci 33(11):1088–1118
Gao D et al (2007) Ultrafine hydrogel nanoparticles: synthetic approach and therapeutic application in living cells. Angew Chem 119(13):2274–2277
Patton JN, Palmer AF (2006) Physical properties of haemoglobin—poly(acrylamide) hydrogel-based oxygen carriers: effect of reaction pH. Langmuir 22(5):2212–2221
Siepmann J, Peppas NA (2012) Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev 64:163–174
Gao D et al (2007) Ultrafine hydrogel nanoparticles: synthetic approach and therapeutic application in living cells. Angewante Chemie 119(13):2274–2277
Patton JN, Palmer AFJL (2006) Physical properties of hemoglobin—poly (acrylamide) hydrogel-based oxygen carriers: effect of reaction pH. Langmui 22(5):2212–2221
Dubrovskii S et al (1990) Comprehensive characterization of superabsorbent polymer hydrogels. Polym Bull 24(1):107–113
Gemeinhart RA et al (2000) pH-sensitivity of fast responsive superporous hydrogels. J Biomater Sci Polym 11(12):1371–1380
Pal K, Banthia AK, Majumdar DKJAP (2007) Preparation and characterization of polyvinyl alcohol-gelatin hydrogel membranes for biomedical applications. AAPS Pharm Sci Tech 8(1):E142–E146
Ganji F, Vasheghani FS, Vasheghani FE (2010) Theoretical description of hydrogel swelling: a review. Iranian Poly J 19(5):375–398
Peppas N et al (2000) Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 50(1):27–46
Lee ALS, Ordoñez GA, Chakraborty SJS (2011) Novel glycerol-crosslinked poly (acr ylic acid) hydrogel for encapsulation and release of benzocaine. Mater Sci 4:81–87
Dhara D, Nisha C, Chatterji PJ (1999) Super absorbent hydrogels: Interpenetrating networks of poly (acrylamide-co-acrylic acid) and poly (vinyl alcohol): Swelling behavior and structural parameters. J Macromol Sci Part A 36(2):197–210
Lee ALS, Ordoñez GA, Chakraborty S (2011) Novel glycerol-crosslinked poly (acr ylic acid) hydrogel for encapsulation and release of benzocaine. Science 4:81–87
Tang Q et al (2009) A multifunctional poly (acrylic acid)/gelatin hydrogel. J Mater Res 24(5):1653–1661
Lalsanati A (2014) Theoretical and experimental investigation of synthesize NiO nanoparticle and nanocomposite; application of them for electrochemical drug analysis. Dissertation for degree, pp 1–50
Desai S, Simonelli A, Higuchi WJ (1965) Investigation of factors influencing release of solid drug dispersed in inert matrices. J Pharm Sci 54(10):1459–1464
Li S et al (2006) A common profile for polymer-based controlled releases and its logical interpretation to general release process. J Pharm Pharm Sci 9(2):238–244
Sharma R, Walker RB, Pathak K (2011) Evaluation of the kinetics and mechanism of drug release from econazole nitrate nanosponge loaded carbapol hydrogel. Indian J Pharm Educ Res 45(1):25–31
Desai SJ et al (1966) Investigation of factors influencing release of solid drug dispersed in inert matrices ii quantitation of procedures. J Pharm Sci 55(11):1224–1229
Ranjha NM, Mudassir J, Zubair SZ (2011) Synthesis and characterization of pH-sensitive pectin/acrylic acid hydrogels for verapamil release study. Iranian Polym J 20(2):147–159
Karadağ E, Saraydın DJPB (2002) Swelling studies of super water retainer acrylamide/crotonic acid hydrogels crosslinked by trimethylolpropane triacrylate and 1, 4-butanediol dimethacrylate. Polym Bull 48(3):299–307
Jiang ZJ (2020) An optimized parameter of hydrogel for a seven-day release of a drug. J Biomater Nanobiotechnol. 11(4):237–244
Anwar M et al (2020) Formulation and evaluation of interpenetrating network of xanthan gum and polyvinylpyrrolidone as a hydrophilic matrix for controlled drug delivery system. Polym Bull 78:59–80
Saidi M, Dabbaghi A, Rahmani SJPB (2019) Swelling and drug delivery kinetics of click-synthesized hydrogels based on various combinations of PEG and star-shaped PCL: influence of network parameters on swelling and release behaviour. Polym Bull 77:3989–4010
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramzan, N., Ranjha, N.M., Hanif, M. et al. Interpenetrating network of gelatin/acrylamide: a binary approach for sustained release and anti-ulcerent effect of RNT. Polym. Bull. 79, 5569–5585 (2022). https://doi.org/10.1007/s00289-021-03831-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03831-6