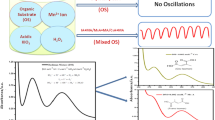
Abstract
The present study pertains to the study of ferroin-catalyzed Belousov–Zhabotinsky (BZ) oscillatory chemical reaction having catechol as an organic substrate in aqueous acid media at 30 ± 0.1 °C under stirred batch conditions. The above system shows oscillations within narrow range of concentration of initial reagents which was monitored potentiometrically in oxidation reduction mode. The system shows a long induction time and a good number of oscillations at 30 ± 0.1 °C. The temperature dependence of the above system was studied. Activation parameters have been calculated, and the results showed good agreement with Arrhenius equation. Thermosensitive polymer, Poly(N-isopropylacrylamide) (PNIPAA), was synthesized by conventional method, and the polymer was characterized by different techniques. The catechol-based BZ system with ferroin as a catalyst was perturbed with N-isopropylacrylamide (NIPAA) monomer and the PNIPAA. Both NIPAA and PNIPAA affected mostly the induction time and number of oscillations. Comparative studies of catechol systems based on Ce(IV) and Mn(II) as metal catalysts were also carried out both in the presence and absence of NIPAA and PNIPAA to explore its oscillatory behavior.
Graphic abstract











Similar content being viewed by others
References
Sciascia L, Rossi F, Sbriziolo C, Liveri MLT, Varsalona R (2010) Oscillatory dynamics of the Belousov-Zhabotinsky system in the presence of a self-assembling nonionic polymer role reactants concentration. Physics Chemistry Chemistry Physics 12:11674–11682
de Groot MSR (1984) Nonequilibrium thermodynamics. Dover
Belousov BP (1959) Periodically acting reaction and its mechanism. Collection of Abstract on Radiation Medicine 1958:145–147
Zhabotinsky AM (1964) Periodic liquid-phase oxidation reactions concentration wave propagation in two-dimensional liquid phase self-oscillating System. Process in Acadamy of Science USSR 157:392–395
Taylor AF (2002) Mechanism and phenomenology of an oscillating chemical reaction. Prog React Kinet Mech 27:247–326
Ganaie NB, Peerzada GM (2009) Effect of initial reagent concentrations on the oscillatory behavior of the BZ reaction in a batch reactor. International Journal of Chemistry Kinetics 41:650–657
Zhabotinsky AM (1964) Periodical oxidation of malonic acid in solution (a study of the Belousov reaction kinetics Biofizika9: 306–311
Zaikin AN, Zhabotinsky AM (1970) Concentration wave propagation in Two-dimensional liquid phase self-oscillating system. Nature 225:535–537
Field RJ, Koros E, Noyes RM (1972) Oscillations in chemical systems. II Thorough analysis of temporal oscillation in the bromate-cerium-malonic acid system. Journal of American Chemistry Society 94:8649–8664
Field RJ, Noyes RM (1974) Oscillations in chemical systems. IV Limit cycle behavior in a model of a real chemical reaction. Journal of Chemistry Physics 60:1877–1884
Field RJ, Burger M (1985) Oscillations and traveling waves in chemical systems. Wiley
Epstein IR, Pojman JA (1998) An introduction to nonlinear chemical dynamics: Oscillations waves, patterns and chaos. Oxford University Press
Gull U, Peerzada GM, Ganaie NB, Dar NA (2013) Effect of initial substrate concentration and temperature on oscillatory behavior of phloroglucinol based BZ reaction. Bull Chem Soc Jpn 86:266–272
Field RJ, Noyes RM (1974) Oscillations in chemical systems. V Quantitative explanation of band migration in the Belousov-Zhabotinskii reaction . Faraday Chemistry Society 9:21–27
Ruoff P (1982) Excitability in a closed stirred Belousov-Zhabotinskii system. Chemistry Physics Letters 90:76–80
Geiseler W, Föllner H (1977) Three steady state situation in an open chemical reaction system. Biophysics Chemistry 6:107–115
Zaikin AN, Zhabotinsky AM (1970) Concentration wave propagation in two-dimensional liquid-phase self-oscillating system. Nature 225:535–537
Winfree AT (1972) Spiral Waves of Chemical Activity. Science 175:634–636
Ruoff P (1993) Excitations induced by fluctuations: An explanation of stirring effects and chaos in closed anaerobic classical Belousov-Zhabotinskii systems. Journal of Physics Chemistry 97:6405–6411
Epstein IR (1995) The consequences of imperfect mixing in autocatalytic chemical and biological system. Nature 374:321–327
Argoul F, Arneodo A, Richetti P, Roux JC, Swinney HL (1987) Chemical chaos: From hints to confirmation. Acc Chem Res 20:436–442
Gao J (2005) Application of oscillating chemical reaction to analytical chemistry: Recent developments. Pak J Biol Sci 8:512–519
Adamĉíková L, Farbulová Z, Sevick P, Kawczynski AL (2003) sequential oscillations in the uncatalyzed bromate oscillator and their simple qualitative model. Journal of Physics Chemistry A 107:508–511
Szabo E, Adamĉíková L, Ševĉík P (2011) Are uncatalyzed bromate oscillators truly gas-free? Journal of Physics Chemistry A 115:6518–6524
Orban M, Koros E, Noyes RM (1979) Chemical oscillations during the uncatalyzed reaction of aromatic compounds with bromate: A plausible skeleton mechanism. Journal of Physics Chemistry 83:3056–3057
Onuma H, Okubo A, Yokokawa AM, Endo M, Kurihashi A, Sawahata H (2011) Rebirth of a dead Belousov-Zhabotinsky oscillator. Journal of Physics Chemistry A 115:14137–14142
Yoshida R (2010) Self-oscillating gels driven by the Belousov-Zhabotinsky reaction as novel smart materials. Adv Mater 22:3463–3483
Mir SH, Nagahara LA, Tundthat T, Tabari PM, Farukawa H, Khosla A (2018) Review—Organic inorganic hybrid functional materials: An integrated platform for applied technologies. Journal of the Electrochemial Society 165:B3137–B3156
Mir SH, Ochiai B (2016) Development of hirarachial polymer @PdNanowire-network synthesis and application as highly active recyclable catalyst and printable conductive ink. ChemistryOpen 5:213–218
Kalmakov A, Klenov DO, Lilach Y, Stemmer S, Maskovits M (2005) Enhanced Gas Sensing by Individual SnO2 Nanowires and Nanobelts Functionalized with Pd Catalyst Particles. Nano Lett 5:667–673
Mir SH, Ochiiai B (2016) Fabrication of polymer Ag- honeycomb hybrid film by metal complexation induced phase separation at the air/water interface thin film. Macromology Materials and Engineering 301:1026–1031
Mir SH, Ochiai B (2018) Conductive polymer Ag- honeycomb thin film: The factors affecting the complexity of microstructure. Journal of Electrochemistry Society 165:B3030–B3034
Mir SH, Hassan PMZ, Danish EY, Aslam M (2020) Pd induced phase separation in poly(methyl methacrylate) telopolymer: Synthesis of nanostructured catalytic Pd nanorods. Colloid Polym Sci 298:441–448
Ozturk T, Savas B, Meyvaci E, Kılıclıoglu A, Hazer B (2020) Synthesis and characterization of the block copolymers using the novel bifunctional initiator by RAFT and FRP technics: Evaluation of the primary polymerization parameters. J Polym Res 27(3):76
Braun D (2009) Origins and development of initiation of free radical polymerization processes International Journal of Polymer Science e893234/1–10
Flory PL (1953) Principles of polymer chemistry. Cornell University Press
Otsu T, Matsumoto A (1994) Macromolecular design: Concepts and practice, Mishra, MK, Ed.; Polymer Frontiers Int.: New York, Chapter 12: 481
Ozturk T, Cakmak I (2010) One-step synthesis of star-block copolymers via simultaneous free radical polymerization of styrene and ring opening polymerization of e-caprolacton using tetrafunctional iniferter. J Appl Polym Sci 117(6):3277–3281
Ozturk T, Cakmak I (2008) One-step synthesis of multiphase block copolymers via simultaneus free radical and ring opening polymerization using poly(ethylene oxide) possessing azo group. J Macromol Sci Part A Pure Appl Chem 45(7):572–577
Ward MA, Georgioc TK (2011) Thermoresponsive polymers for biomedical applications. Polymers 3:1215–1242
Shimizu K, Fujita H, Nagamori E (2010) Oxygen plasma-treated thermoresponsive polymer surfaces for cell sheet engineering. Biotechnology Bioengineering 106:303–310
Twaites BR, Alarcon CD, Lavigne M, Saulnier A, Pennadam SS, Cunliffe DD, Gorecki C, Alexander C (2005) Thermoresponsive polymers as gene delivery vectors: Cell viability DNA transport and transfection studies. Journal of Control Release 108:472–483
Doorty KB, Golubeva TA, Gorelov AV, Rochev YA, Allen LT, Dawson KA, Gallagher KWM (2003) Poly(N-isopropylacrylamide) co-polymer films as potential vehicles for delivery of an antimitotic agent to vascular smooth muscle cells. Cardiovasc Pathol 12:105–110
Stile RA, Healy KE (2001) Thermo-responsive peptide-modified hydrogels for tissue regeneration. Biomacromol 2:185–194
Hacker MC, Klouda L, Ma BB, Kretlow JD, Mikos AG (2008) Synthesis and characterization of injectable, thermally and chemically gelable, amphiphilic poly(N-isopropylacrylamide)-based macromers. Biomacromol 9:1558–1570
Feil H, Bae YH, Feijen J, Kim SW (1993) Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 26:2496–2500
Vihola H, Laukkanen A, Tenhu H, Hirvonen J (2008) Drug release characteristics of physically cross-linked thermosensitive poly(N-vinylcaprolactam) hydrogel particles. J Pharm Sci 97:4783–4793
Vihola H, Marttila AK, Pakkanen JS, Andersson M, Laukkanen A, Kaukonen H, Tenhu AM, Hirvonen J (2007) Cell-polymer interactions of fluorescent polystyrene latex particles coated with thermosensitive poly(N-isopropylacrylamide) and poly(N-vinylcaprolactam) or grafted with poly(ethylene oxide)-macromonomer. Int J Pharm 343:238–246
Pasparakis G, Vamvakaki M (2011) Multiresponsive polymers:Nano-sized assemblies. Polymer Chemistry 2:1234–1248
Liu FM, Urban W (2010) Recent advances and challenges in designing stimuli-responsive polymers. Prog Polym Sci 35:3–23
Shah IA, Peerzada GM, Ganaie NB, Dar NA (2012) A Kinetic Study on Catechol Based Belousov-Zhabotinsky Reaction. Int J Chem Kinet 45:141–151
Feil H, Bae YH, Feijen J, Kim SW (1993) Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 1(26):2496–2500
Pasparakis G, Vamvakaki M (2011) Multiresponsive polymers: Nano-sized assemblies, stimuli-sensitive gels and smart surfaces. Polymer Chemistry 2:234–1248
Dutt AK, Banerjee RS (1985) Behaviour of a BZ oscillating system at low temperatures. Chemistry Physics Letters 51:186–188
Bala D, Treindl L (2000) Temperature and oxygen effect on oscillations of the belousov-zhabotinsky reaction with 2-oxopentanedioic acid as substrate czech. Chem Commun 65:1839–1847
Vilcu R, & Bala D (2002) Chemical oscillations in homogenous systems 1. Essential thermodynamic and kinetic conditions for the occurance of oscillations An Uni Bucuresti ChimXII: I-II 303–308
Nath MA, Ganaie NB, Rastogi RP, Peerzada GM (2008) Effect of temperature on oscillatory behaviour of the system containing isomers of hydroxybenzoic acid in batch reactor. E-Journal of Chemistry 5(4):832–837
Nagy G, Koros E, Oftedal N, Tjelflaat K, Rouff P (1996) Effect of temperature in cerium-ion-catalyzed bromate-driven oscillators. Chemistry Physics Letters 250:255–260
Nagao R, Epstein IR, Gonzalez ER, Varela HJ (2008) Temperature (over) compensation in an oscillatory surface reaction. Journal of Physics Chemistry A 112(20):4617–4624
Kovacs KM, Rabai G (2002) Temperature compensation in pH oscillators. Physics Chemistry Chemistry Physics 4:5265–5269
Rabai G, Hanazaki I (1999) Temperature compensation in the oscillatory hydrogen peroxide-thiosulfate-sulfite flow system. Chem Commun 19:1965–1966
Challis BC, Challis J (1970) The chemistry of amides Interscience Publ. Wiley
Uflyand IE, Starikov AG, Savostyanov VS, Pomogailo AD (1990) Preparation and reactivity of metal-containing monomers. 17. Spatial and electronic structure of complexes of cobalt nitrate and chloride with acrylamide. Bulletin of the Academy of Sciences of the USSR, Division of Chemical Science 39:1185–1189
Farona MF, Ayers WT, Ramsey GB, Grasselli JG (1969) Acrylamide and N, N-dimethylacrylamide complexes. II N-protonation and N-bonding to some transition metal perchlorates and tetrafluouroborates. Inorg Chim Acta 3:503–507
Kanazawa H, Kashiwase Y, Yamamoto K, Matsushima M, Kikushi Y, Sakurai A, Okano T (1997) Temperature-responsive liquid chromatography. 2 effects of hydrophobic groups in N-isopropylacrylamide copolymer-modified silica. Anal Chem 69:823–830
Chung JE, Yokoyama M, Yamato M, Aoyagi T, Sakurai Y, Okano T (1999) Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly(N-isopropylacrylamide) and poly(butylmethacrylate). Journal of Control Release 62:115–127
Okano T, Yamada N, Okuhara M, Sakai H, Sakurai Y (1995) Mechanism of cell detachment from temperature-modulated, hydrophilic–hydrophobic polymer surfaces. Biomaterials 16:297–303
Schild HG, Tirrell DA (1990) Microcalorimetric detection of lower critical solution temperatures in aqueous polymer solutions. J Phys Chem 94:4352–4435
Acknowledgements
The authors acknowledge the Head Department of Chemistry for providing infrastructural support for carrying out this work.
Funding
The authors acknowledge the financial support in the form of Major Research Project No. SB/S1/PC-023/2014 sponsored by SERB, Govt. of India New Delhi for carrying out this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lateef, S., Ganaie, N.B. & Peerzada, G.M. Perturbation on dynamics of ferroin-catalyzed Belousov–Zhabotinsky reaction by monomer N-isopropylacrylamide and poly(N-isopropylacrylamide). Polym. Bull. 79, 2777–2798 (2022). https://doi.org/10.1007/s00289-021-03660-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03660-7