Abstract
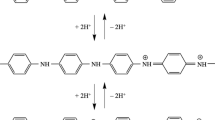
Polyaniline (PAni) has been used widely in sensor applications. However, it has the disadvantage of being insoluble in water. To overcome this limitation, aniline (Ani) monomer was polymerized in the presence of water-soluble cellulose to produce a complex. In this research work, water-soluble PAni-cellulose derivatives were prepared and developed as a chemical sensor for detecting hydrazine in aqueous effluents. Three cellulose derivatives methylcellulose (MC), hydroxypropyl cellulose (HPC), and hydroxypropyl methylcellulose (HPMC) were incorporated during the polymerization of Ani through chemical oxidation method to produce PAni-MC, PAni-HPC, and PAni-HPMC derivative, respectively. The electrical conductivities of these PAni-cellulose derivatives were affected by the presence of hydrazine at different ppm levels. These offer the potential of application as a chemical sensor for hydrazine in the commercial wastewater. The sensor responses of PAni-cellulose derivatives toward hydrazine were observed through the electrical conductivity values. Results have shown that PAni-MC could attain the highest sensitivity of 0.0143 ppm−1, followed by PAni-HPC that has acquired sensitivity of 0.0052 ppm−1. Meanwhile, PAni-HPMC has recorded the lowest sensitivity of 0.0045 ppm−1.









Similar content being viewed by others
References
Harun MH, Saion E, Kassim A, Yahya N, Mahmud E (2007) Conjugated conducting polymers: A brief overview. J Am Stat Assoc 2:63–68
Seitz WR (1984) Chemical sensors based on fiber optics. Anal Chem 56:16–34
Wen W (2016) Introductory chapter: what is chemical sensor? progresses in chemical sensor. Intech Open. https://doi.org/10.5772/64626
Li X, Wang Y, Yang X, Chen J, Fu H, Cheng T (2012) Conducting polymers in environmental analysis. Trends Anal Chem 39:163–179
Sambasevam KP, Mohamad S, Phang SW (2015) Effect of dopant concentration on polyaniline for hydrazine detection. Mater Sci Semicond Process 33:24–31
Lange U, Roznyatovskaya NV, Mirsky VM (2008) Conducting polymers in chemical sensors and arrays. Anal Chim Acta 614:1–26
Weiller BH, Virji S, Baker C, Huang J, Li D, Kaner RB (2005) Polyaniline nanofibers and composite materials for chemical detection. NSTI-Nanotech 2:1–4
Sen T, Mishra S, Shimpi NG (2017) A β-cyclodextrin based binary dopant for polyaniline: Structural, thermal, electrical, and sensing performance. Mater Sci and Eng B 220:13–21
Wang X, Meng S, Tebyetekerwa M, Weng W, Pionteck J, Sun B, Qin Z, Zhu M (2017) Nanostructured polyaniline/poly(styrene-butadiene-styrene) composite fiber for use as highly sensitive and flexible ammonia sensor. Synth Met 233:86–93
Shukla SK (2012) Synthesis of polyaniline grafted cellulose suitable for humidity sensing. Indian J Eng Mater Sci 19:417–420
Casado UM, Quintanilla RM, Aranguren MI, Marcovich NE (2012) Composite films based on shape memory polyurethanes and nanostructured polyaniline or cellulose-polyaniline particles. Synth Met 162:1654–1664
Jia B, Hino T, Kuramoto N (2007) Synthesis and chiroptical properties of water processable polyaniline using methylcellulose as a molecular template. React Funct Polym 67:836–843
Luong ND, Korhonen JT, Soininen AJ, Ruokolainen J, Johansson LS, Seppala J (2013) Processable polyaniline suspensions through in situ polymerization onto nanocellulose. Eur Polym J 49:335–344
Arulraj AD, Vijayan M, Vasantha VS (2015) Spectrophotometric determination of pico- molar level of hydrazine by using Alizarin red in water and urine samples. Spectrochim Acta Part A: Mol and Biomol Spectrosc 148:355–361
Zhou T, Lu P, Zhang Z, Wang Q, Umar A (2016) Perforated Co3O4 nanoneedles assembled in chrysanthemum-like Co3O4 structures for ultra-high sensitive hydrazine chemical sensor. Sens and Actuators B: Chem 235:457–465
Dong Y, Yang Z, Sheng Q, Zheng J (2018) Solvothermal synthesis of Ag@Fe3O4 nanosphere and its application as hydrazine sensor. Colloids and Surf A: Physicochem and Eng Asp 538:371–377
Sakthinathan S, Kubendhiran S, Chen SM, Govindasamy M, Al-Hemaid FMA, Ajmal Ali M, Tamizhdurai P, Sivasanker S (2016) Metallated porphyrin noncovalent interaction with reduced graphene oxide-modified electrode for amperometric detection of environmental pollutant hydrazine. Appl Organomet Chem 2017:e3703
Habibi E (2019) Mesoporous Pd ǀ β-SiCNW-nC based home made screen printed electrode for high sensitive detection of hydrazine. Microchem J 149:104004
Xingzong J, Zhen L, Mingqin S, Sili Y, Xiaoyang Z, Yongle Z, Linxi H (2020) A fluorescence “turn-on” sensor for detecting hydrazine in environment. Microchem J 152:104376
Teng M, Zhou Z, Qin Y, Zhao Y, Zhao C, Cao J (2020) A water-soluble fluorescence sensor with high specificity for detecting hydrazine in river water detection and A549 cell imaging. Sens and Actuators B: Chem 311:127914
Rahy A, Rguig T, Cho SJ, Bunker CE, Yang DJ (2011) Polar solvent soluble and hydrogen absorbing polyaniline nanofibers. Synth Met 161:280–284
Hussin H, Gan SN, Mohamad S, Phang SW (2017) Synthesis of water-soluble polyaniline by using different types of cellulose derivatives. Polym Polym Compos 25:515–520
Barik A, Solanki PR, Kaushik A, Ali A, Pandey MK, Kim CG, Malhotra BD (2010) Polyaniline-carboxymethyl cellulose nanocomposite for cholesterol detection. J Nanosci Nanotechnol 10:1–10
Chattopadhyay D, Mandal BM (1996) Methyl cellulose stabilized polyaniline dispersions. Langmuir 12:1585–1588
Hino T, Namiki T, Kuramoto N (2006) Synthesis and characterization of novel conducting composites of polyaniline prepared in the presence of sodium dodecylsulfonate and several water soluble polymers. Synth Met 156:1327–1332
Shao L, Qiu J, Liu M, Feng H, Lei L, Zhang G, Zhao Y, Gao C, Qin L (2011) Synthesis and characterization of water-soluble polyaniline films. Synth Met 161:806–811
Yuan GL, Kuramoto N (2002) Water-processable chiral polyaniline derivatives doped and intertwined with dextran sulfate: synthesis and chiroptical properties. Macromol 35:9773–9779
Laska J (2004) Conformations of polyaniline in polymer blends. J of Mol Struct 701:13–18
Patil R, Harima Y, Yamashita K, Komaguchi K, Itagaki Y, Shiotani M (2002) Charge carriers in polyaniline film: A correlation between mobility and in-situ ESR measurements. J Electroanal Chem 518:13–19
Xia Y, Wiesinger JM, MacDiarmid AG, Epstein AJ (1995) Camphorsulfonic acid fully doped polyaniline emeraldine salt: Conformations in different solvents studied by an ultraviolet/visible/near-infrared spectroscopic method. Chem of Mater 7:443–445
Bin L, Konecke S, Wegiel LA, Taylor LS, Edgar KJ (2013) Both solubility and chemical stability of curcumin are enhanced by solid dispersion in cellulose derivatives matrices. Carbohydr Polym 98:1108–1116
Chattopadhyay D, Bain MK (2008) Electrically conductive nanocomposites of polyaniline with poly(vinyl alcohol) and methylcellulose. J Appl Polym Sci 110:2849–2853
Gosh M, Barman A, Meikap AK, De SK, Chatterjee S (1999) Hopping transport in HCl doped conducting polyaniline. Phys Lett A 260:138–148
Phasuksom K, Prissanaroon-Ouajai W, Sirivat A (2018) Electrical conductivity response of methanol sensor based on conductive polyindole. Sens Actuators B 262:1013–1023
Virji S, Kaner RB, Weiller BH (2005) Hydrazine detection by polyaniline using fluorinated alcohol additives. Chem Mater 17:1256–1260
Bochek AM (2003) Effect of hydrogen bonding on cellulose solubility in aqueous and nonaqueous solvents. Russ J Appl Chem 76:1711–1719
Tayeb AH, Amini E, Ghasemi S, Tajvidi M (2018) Cellulose nanomaterials-binding properties and applications: A review. Mol 23:1–24
Bodvik R, Dedinaite A, Karlson L, Bergstrom M, Baverback P, Pedersen JS, Edwards K, Karlsson G, Varga I, Claesson PM (2010) Aggregation and network formation of aqueous methylcellulose and hydroxypropylmethylcellulose solutions. Colloids Surf A: Physicochem Eng Asp 354:162–171
Li B, Santhanam S, Schultz L, Jeffries-EL M, Iovu MC, Sauve G, Cooper J, Zhang R, Revelli JC, Kusne AG, Snyder JL, Kowalewski T, Weiss LE, McCullough RD, Fedder GK, Lambeth DN (2007) Inkjet printed chemical sensor array based on polythiophene conductive polymers. Sens Actuators B 123:651–660
Acknowledgements
The authors would like to acknowledge the University of Malaya through the following grants; UMRG (RG271-13AFR) and PPP (PG198-2016A), Tunku Abdul Rahman University College, and the Ministry of Higher Education Malaysia (MOHE) for their financial support and scholarship (MyMaster) to complete this research study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hussin, H., Gan, SN. & Phang, SW. Effect of functional groups in the PAni-cellulose derivatives-based sensor in hydrazine detection. Polym. Bull. 79, 1843–1856 (2022). https://doi.org/10.1007/s00289-021-03589-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03589-x