Abstract
The present study describes a new approach for the lead (II)-imprinted interpenetrating polymer linkage synthesis by ion-imprinted polymers. The development of lead (II) (Pb2+) ion-imprinted polymer by employing the 4-vinyl pyridine as a complexing agent and methacrylic acid as a functional monomer was used for the selective elimination of noxious Pb2+ ions from an aqueous environment. Different analytical techniques have been used for the characterization of synthesized Pb2+ion-imprinted polymeric material such as scanning electron microscopy, energy-dispersive X-ray, and Fourier-transform infrared spectroscopy. During adsorption study, different parameters have been optimized such as pH, agitation time, time study, and adsorbent dose to achieve maximum adsorption capacity. This study well fitted the Langmuir isotherm model, while the kinetic study was well defined by pseudo-second order. The relative selective factor (Kʹ) of Pb2+ ion and coexisting ions was greater than 1 due to the imprinting effect. The maximum adsorption capability of Pb2+ ion-imprinted polymer was 85.47 mg g−1 at pH 6. The developed method obtained a good linear range from 10 to 100 μg L−1 concentration, with a limit of detection (0.74 µg L−1) and a limit of quantification (2.48 µg L−1). The developed methodology was validated by the spiking addition method and obtained good results in accordance with spiking values in real samples.
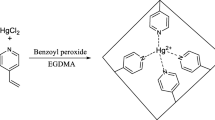
Graphic abstract
Graphical view of lead (II)-imprinted interpenetrating polymer linkage
















Similar content being viewed by others
References
Ansari R, Khoshbakht FN, Fallah DA (2009) Removal of thiocyanate ions from aqueous solutions using polypyrrole and polyaniline conducting electroactive polymers. J Iran Chem Res 2:163–171
Bhaumik M, Maity A, Srinivasu V, Onyango MS (2012) Removal of hexavalent chromium from aqueous solution using polypyrrole-polyaniline nanofibers. Chem Eng J 181:323–333
Tuzen M, Soylak M, Parlar K (2005) Cadmium and lead contamination in tap water samples from Tokat, Turkey. Bull Environ Contam Toxicol 75(2):284–289
Singh A, Sharma RK, Agrawal M, Marshall FM (2010) Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem Toxicol 48(2):611–619
Grahek Ž, Mačefat MR, Lulić S (2006) Isolation of lead from water samples and determination of 210Pb. Anal Chim Acta 560(1–2):84–93
Gama EM, da Silva LA, Lemos VA (2006) Preconcentration system for cadmium and lead determination in environmental samples using polyurethane foam/Me-BTANC. J Hazard Mater 136(3):757–762
Bagal-Kestwal D, Karve MS, Kakade B, Pillai VK (2008) Invertase inhibition based electrochemical sensor for the detection of heavy metal ions in aqueous system: application of ultra-microelectrode to enhance sucrose biosensor’s sensitivity. Biosens Bioelectron 24(4):657–664
Vivier J, Ehlers M, Grabow W (2004) Detection of enteroviruses in treated drinking water. Water Res 38(11):2699–2705
Gao C, Yu X-Y, Xiong S-Q, Liu J-H, Huang X-J (2013) Electrochemical detection of arsenic (III) completely free from noble metal: Fe3O4 microspheres-room temperature ionic liquid composite showing better performance than gold. Anal Chem 85(5):2673–2680
Turdean GL (2011) Design and development of biosensors for the detection of heavy metal toxicity. Int J Electrochem 2011:5
Ettinger AS, Wengrovitz AM (2010) Guidelines for the identification and management of lead exposure in pregnant and lactating women
Zhang R, Wilson VL, Hou A, Meng G (2015) Source of lead pollution, its influence on public health and the countermeasures. Int J Health Anim Sci Food Saf 2(1):18–31
Gerçel Ö, Gerçel HF (2007) Adsorption of lead (II) ions from aqueous solutions by activated carbon prepared from biomass plant material of Euphorbia rigida. Chem Eng J 132(1–3):289–297
Rubio J, Souza M, Smith R (2002) Overview of flotation as a wastewater treatment technique. Miner Eng 15(3):139–155
Emamjomeh MM, Sivakumar M (2009) Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. J Environ Manage 90(5):1663–1679
Zaijun L, Yuling Y, Jian T, Jiaomai P (2003) Spectrophotometric determination of trace lead in water after preconcentration using mercaptosephadex. Talanta 60(1):123–130
Karve M, Rajgor RV (2007) Solid phase extraction of lead on octadecyl bonded silica membrane disk modified with Cyanex302 and determination by flame atomic absorption spectrometry. J Hazard Mater 141(3):607–613
Silva EL, dos Santos RP (2009) Simultaneous flow injection preconcentration of lead and cadmium using cloud point extraction and determination by atomic absorption spectrometry. J Hazard Mater 161(1):142–147
Zheng F, Hu B (2007) MPTS-silica coated capillary microextraction on line hyphenated with inductively coupled plasma atomic emission spectrometry for the determination of Cu, Hg and Pb in biological samples. Talanta 73(2):372–379
Xia L, Li X, Wu Y, Hu B, Chen R (2008) Ionic liquids based single drop microextraction combined with electrothermal vaporization inductively coupled plasma mass spectrometry for determination of Co, Hg and Pb in biological and environmental samples. Spectrochim Acta Part B 63(11):1290–1296
Rammika M (2010) An ion imprinted polymer for the determination of Ni (II) ions from mine tailing samples. Rhodes University, Grahamstown
Ghaedi M, Ahmadi F, Tavakoli Z, Montazerozohori M, Khanmohammadi A, Soylak M (2008) Three modified activated carbons by different ligands for the solid phase extraction of copper and lead. J Hazard Mater 152(3):1248–1255
Soylak M, Tuzen M, Narin I (2006) Solid phase extraction of iron and lead in environmental matrices on amberlite xad-1180/pv. Química Nova 29(2):203–207
Jawad AH, Abdulhameed AS, Mastuli MS (2020) Acid-factionalized biomass material for methylene blue dye removal: a comprehensive adsorption and mechanism study. J Taibah Univ Sci 14(1):305–313
Jawad AH, Abdulhameed AS, Mastuli MS (2020) Mesoporous crosslinked chitosan-activated charcoal composite for the removal of thionine cationic dye: comprehensive adsorption and mechanism study. J Polym Environ 28(3):1095–1105
Jawad AH, Mubarak NSA, Abdulhameed AS (2020) Tunable Schiff’s base-cross-linked chitosan composite for the removal of reactive red 120 dye: adsorption and mechanism study. Int J Biol Macromol 142:732–741
Jawad AH, Abd Malek NN, Abdulhameed AS, Razuan R (2020) Synthesis of magnetic chitosan-fly Ash/Fe3O4 composite for adsorption of reactive orange 16 dye: optimization by Box–Behnken design. J Polym Environ 28(3):1068–1082
Kempe M, Mosbach K (1995) Molecular imprinting used for chiral separations. J Chromatogr A 694(1):3–13
Nishide H, Deguchi J, Tsuchida E (1976) Selective adsorption of metal ions on crosslinked poly (vinylpyridine) resin prepared with a metal ion as a template. Chem Lett 5(2):169–174
Whitcombe MJ, Rodriguez ME, Villar P, Vulfson EN (1995) A new method for the introduction of recognition site functionality into polymers prepared by molecular imprinting: synthesis and characterization of polymeric receptors for cholesterol. J Am Chem Soc 117(27):7105–7111
Dahdouh N, Amokrane S, Murillo R, Mekatel E, Nibou D (2020) Removal of methylene blue and basic yellow 28 dyes from aqueous solutions using sulphonated waste poly methyl methacrylate. J Polym Environ 28(1):271–283
Li Z, Li J, Wang Y, Wei Y (2014) Synthesis and application of surface-imprinted activated carbon sorbent for solid-phase extraction and determination of copper (II). Spectrochim Acta Part A Mol Biomol Spectrosc 117:422–427
Jagirani MS, Balouch A, Mahesar SA, Kumar A, Mustafai FA, Bhanger MI (2019) Preparation of novel arsenic-imprinted polymer for the selective extraction and enhanced adsorption of toxic As3+ ions from the aqueous environment. Polymer Bull 77:1–19
Mustafai FA, Balouch A, Jalbani N, Bhanger MI, Jagirani MS, Kumar A, Tunio A (2018) Microwave-assisted synthesis of imprinted polymer for selective removal of arsenic from drinking water by applying Taguchi statistical method. Eur Polymer J 109:133–142
Kumar A, Balouch A, Pathan AA (2019) Synthesis, adsorption and analytical applicability of Ni-imprinted polymer for selective adsorption of Ni2+ ions from the aqueous environment. Polymer Test 77:105871
Kumar A, Balouch A, Pathan AA, Abdullah, Jagirani MS, Mahar AM, Rajput M-U-H (2019) Novel chromium imprinted polymer: synthesis, characterization and analytical applicability for the selective remediation of Cr (VI) from an aqueous system. Int J Environ Anal Chem 99(5):454–473
Kumar S, Alveroglu E, Balouch A, Talpur FN, Jagirani MS, Mahar AM, Pato AH, Mal D, Lal S (2020) Fabrication of chromium imprinted polymer: A real magneto selective sorbent for chromium Cr (VI) removal in a real water sample. New J Chem 43:18668–18678
Alveroglu E, Balouch A, Talpur FN, Shah MT, Kumar A, Mahar AM, Jagirani MS (2020) Ultrasonic mediated synthesis of arsenic imprinted polymer and their analytical practicality as a selective sorbent for removal of toxic As3+ ion from real samples. J Polym Res 27(9):1–11
Jagirani MS, Balouch A, Mahesar SA, Kumar A, Baloch AR, Bhanger MI (2020) Fabrication of cadmium tagged novel ion imprinted polymer for detoxification of the toxic Cd2+ ion from aqueous environment. Microchem J 158:105247
Qiao F, Sun H, Yan H, Row KH (2006) Molecularly imprinted polymers for solid phase extraction. Chromatographia 64(11–12):625–634
Pan J, Wang S, Zhang R (2006) A novel Pb (II)-imprinted IPN for selective preconcentration of lead from water and sediments. Int J Environ Anal Chem 86(11):855–865
Balouch A, Talpur FN, Kumar A, Shah MT, Mahar AM (2019) Synthesis of ultrasonic-assisted lead ion imprinted polymer as a selective sorbent for the removal of Pb2+ in a real water sample. Microchem J 146:1160–1168
Hande PE, Kamble S, Samui AB, Kulkarni PS (2016) Chitosan-based lead ion-imprinted interpenetrating polymer network by simultaneous polymerization for selective extraction of lead (II). Ind Eng Chem Res 55(12):3668–3678
Behbahani M, Bagheri A, Taghizadeh M, Salarian M, Sadeghi O, Adlnasab L, Jalali K (2013) Synthesis and characterisation of nano structure lead (II) ion-imprinted polymer as a new sorbent for selective extraction and preconcentration of ultra trace amounts of lead ions from vegetables, rice, and fish samples. Food Chem 138(2–3):2050–2056
Luo X, Liu L, Deng F, Luo S (2013) Novel ion-imprinted polymer using crown ether as a functional monomer for selective removal of Pb (II) ions in real environmental water samples. J Mater Chem A 1(28):8280–8286
Cai X, Li J, Zhang Z, Yang F, Dong R, Chen L (2013) Novel Pb2+ ion imprinted polymers based on ionic interaction via synergy of dual functional monomers for selective solid-phase extraction of Pb2+ in water samples. ACS Appl Mater Interfaces 6(1):305–313
Tarley CRT, Andrade FN, De Santana H, Zaia DAM, Beijo LA, Segatelli MG (2012) Ion-imprinted polyvinylimidazole-silica hybrid copolymer for selective extraction of Pb (II): characterization and metal adsorption kinetic and thermodynamic studies. React Funct Polym 72(1):83–91
Zhu X, Cui Y, Chang X, Zou X, Li Z (2009) Selective solid-phase extraction of lead (II) from biological and natural water samples using surface-grafted lead (II)-imprinted polymers. Microchim Acta 164(1–2):125–132
Acknowledgements
This research work is a part of my PhD thesis that was submitted to the higher education commission of Pakistan performed at the National Center of Excellence in Analytical Chemistry University of Sindh Jamshoro. The authors greatly acknowledge the scholarship support from the Scientific and Technological Research Council of Turkey (TUBITAK-2221) Visiting Scientist Program for International Citizens. The authors are thankful for support and fund by Pakistan Science Foundation, Pakistan, under research Grant No. PSF/Res/S-SU/Chem (465).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jagirani, M.S., Balouch, A., Mahesar, S.A. et al. Selective and sensitive detoxification of toxic lead ions from drinking water using lead (II) ion-imprinted interpenetrating polymer linkage. Polym. Bull. 79, 1887–1909 (2022). https://doi.org/10.1007/s00289-021-03546-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03546-8