Abstract
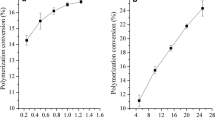
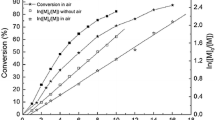
In this paper, the polymerization of acrolein (A) via UV-mediated atom transfer radical polymerization (ATRP) is reported. The optimization of the experimental conditions of the polymerization is investigated, and it shows that dimethyl sulfoxide as solvent, ethyl 2-bromoisobutyrate (EBIB) and fluorescein (FL) as catalyst, and [A]0/[EBIB]0/[FL]0 = 200/1/0.1 in the period of 5 h at 47 °C are suitable conditions for the reaction. In this way, the yield of the polymer is 24.5%. The glass transition temperature and melting point of polyacrolein characterized by differential scanning calorimetry are 115.5 °C and 165.7 °C, respectively. At low conversion, the polymerization conforms to be the first-order kinetics reaction. The dependence of polymerization on light source is proved by “on/off” light source experiment. In short, this study opens up a new way for the ATRP of acrolein, and the polyacrolein with abundant aldehyde groups can be used in the fields of biomedical labeling, immobilization carrier and adsorption of organic amines.
Graphic abstract






Similar content being viewed by others
References
Slomkowski S (1998) Polyacrolein containing microspheres: Synthesis, properties and possible medical applications. Prog Polym Sci 23(5):815–874. https://doi.org/10.1016/S0079-6700(97)00053-1
Savoldelli A, Magna G, Di Natale C, Catini A, Nardis S, Fronczek FR, Smith KM, Paolesse R (2017) β-acrolein-substituted corroles: a route to the preparation of functionalized polyacrolein microspheres for chemical sensor applications. Chem Eur J 23(59):14819–14826. https://doi.org/10.1002/chem.201702380
Rembaum A, Chang M, Richards G, Li M (1984) Structure and immunological properties of polyacrolein formed by means of ionizing radiation and base catalysis. J Polym Sci Pol Chem 22(3):609–621. https://doi.org/10.1002/pol.1984.170220311
Schulz VRC, Kern W (1956) Polymere acroleine, 2. Mitt. reaktionen und konstitution des disacryls. Macromol Chem Phys 18(1):4–8. https://doi.org/10.1002/macp.1956.020180102
Schulz RC, Cherdron H, Kern W (1957) Die redox-polymerisation des acroleins inwäßrigem medium. polymere acroleine. 6. mitteilung. Macromol Chem Phys 24(1):141–151. https://doi.org/10.1002/macp.1957.020240110
Schulz VRC, Kern W (1959) Molgewichtsbestimmungen an polyacrolein-thiophenolmercaptalen. polymere acroleine, 13. mitteilung. Macromol Chem Phys 30(1):39–47. https://doi.org/10.1002/macp.1959.020300103
Schulz VRC, Suzuki S, Cherdron H, Kern W (1962) Die radikalinduzierte polymerisation von acrolein und α-methylacrolein in dimethylformamid. polymere acroleine. 22. Mitt. 1. Macromol Chem Phys 53(1):145–153. https://doi.org/10.1002/macp.1962.020530115
Blacet FE, Fielding GH, Roof JG (1937) The photolysis of the aliphatic aldehydes. V. acrolein. J Am Chem Soc 59(11):2375–2379
Kumakura M, Suzuki M, Kaetsu I (1984) Properties of functional polymeric microspheres obtained by radiation polymerization of acrolein. J Colloid Interface Sci 97(1):157–165. https://doi.org/10.1016/0021-9797(84)90283-2
Schulz VRC (1955) Polymere acroleine, I. Mitt. untersuchungen uber die polymerisation des acroleins. Macromol Chem Phys 17(1):62–73. https://doi.org/10.1002/macp.1955.020170107
Li Y-H, Chen Y-C (2020) Triphenylamine-hexaarylbiimidazole derivatives as hydrogen-acceptor photoinitiators for free radical photopolymerization under UV and LED light. Polym Chem 11(8):1504–1513. https://doi.org/10.1039/c9py01605h
Karabulut HRF, Mert B, Altinkok C, Karatavuk AO, Acik G, Turkyilmaz M (2020) Synthesis of new bio-based hydrogels derived from bile acids by free-radical photo-polymerization. Polym Adv Technol. https://doi.org/10.1002/pat.5077
Liu B, Zhang Y-Y, Zhang X-H, Du B-Y, Fan Z-Q (2016) Fixation of carbon dioxide concurrently or in tandem with free radical polymerization for highly transparent polyacrylates with specific UV absorption. Polym Chem 7(22):3731–3739. https://doi.org/10.1039/c6py00525j
Matyjaszewski K (2012) Atom transfer radical polymerization (ATRP): current status and future perspectives. Macromolecules 45(10):4015–4039. https://doi.org/10.1021/ma3001719
Pan X, Fantin M, Yuan F, Matyjaszewski K (2018) Externally controlled atom transfer radical polymerization. Chem Soc Rev 47(14):5457–5490. https://doi.org/10.1039/c8cs00259b
Treat NJ, Sprafke H, Kramer JW, Clark PG, Barton BE, Read de Alaniz J, Fors BP, Hawker CJ (2014) Metal-free atom transfer radical polymerization. J Am Chem Soc 136(45):16096–16101. https://doi.org/10.1021/ja510389m
Dadashi-Silab S, Pan X, Matyjaszewski K (2017) Phenyl benzo[b]phenothiazine as a visible light photoredox catalyst for metal-free atom transfer radical polymerization. Chemistry 23(25):5972–5977. https://doi.org/10.1002/chem.201605574
Theriot JC, Lim CH, Yang H, Ryan MD, Musgrave CB, Miyake GM (2016) Organocatalyzed atom transfer radical polymerization driven by visible light. Science 352(6289):1082. https://doi.org/10.1126/science.aaf3935
Cole JP, Federico CR, Lim CH, Miyake GM (2019) Photoinduced organocatalyzed atom transfer radical polymerization using low ppm catalyst loading. Macromolecules 52(2):747–754. https://doi.org/10.1021/acs.macromol.8b02688
Miyake GM, Theriot JC (2014) Perylene as an organic photocatalyst for the radical polymerization of functionalized vinyl monomers through oxidative quenching with alkyl bromides and visible light. Macromolecules 47(23):8255–8261. https://doi.org/10.1021/ma502044f
Aydogan C, Yilmaz G, Yagci Y (2017) Synthesis of hyperbranched polymers by photoinduced metal-free ATRP. Macromolecules 50(23):9115–9120. https://doi.org/10.1021/acs.macromol.7b02240
Allushi A, Jockusch S, Yilmaz G, Yagci Y (2016) Photoinitiated metal-free controlled/living radical polymerization using polynuclear aromatic hydrocarbons. Macromolecules 49(20):7785–7792. https://doi.org/10.1021/acs.macromol.6b01752
Pearson RM, Lim CH, McCarthy BG, Musgrave CB, Miyake GM (2016) Organocatalyzed atom transfer radical polymerization using N-aryl phenoxazines as photoredox catalysts. J Am Chem Soc 138(35):11399–11407. https://doi.org/10.1021/jacs.6b08068
Ryan MD, Pearson RM, French TA, Miyake GM (2017) Impact of light intensity on control in photoinduced organocatalyzed atom transfer radical polymerization. Macromolecules 50(12):4616–4622. https://doi.org/10.1021/acs.macromol.7b00502
Liu X, Zhang L, Cheng Z, Zhu X (2016) Metal-free photoinduced electron transfer–atom transfer radical polymerization (PET–ATRP) via a visible light organic photocatalyst. Polym Chem 7(3):689–700. https://doi.org/10.1039/c5py01765c
Kutahya C, Aykac FS, Yilmaz G, Yagci Y (2016) LED and visible light-induced metal free ATRP using reducible dyes in the presence of amines. Polym Chem 7(39):6094–6098. https://doi.org/10.1039/c6py01417h
Lim CH, Ryan MD, McCarthy BG, Theriot JC, Sartor SM, Damrauer NH, Musgrave CB, Miyake GM (2017) Intramolecular charge transfer and ion pairing in N, N-diaryl dihydrophenazine photoredox catalysts for efficient organocatalyzed atom transfer radical polymerization. J Am Chem Soc 139(1):348–355. https://doi.org/10.1021/jacs.6b11022
Niu T, Jiang J, Li S, Ni B, Liu X, Chen M (2017) Well-defined high-molecular-weight polyacrylonitrile formation via visible-light-induced metal-free radical polymerization. Macromol Chem Phys 218(15):1700169. https://doi.org/10.1002/macp.201700169
Allushi A, Kutahya C, Aydogan C, Kreutzer J, Yilmaz G, Yagci Y (2017) Conventional type II photoinitiators as activators for photoinduced metal-free atom transfer radical polymerization. Polym Chem 8(12):1972–1977. https://doi.org/10.1039/c7py00114b
Barner-Kowollik C, Davis TP, Stenzel MH (2004) Probing mechanistic features of conventional, catalytic and living free radical polymerizations using soft ionization mass spectrometric techniques. Polymer 45(23):7791–7805. https://doi.org/10.1016/j.polymer.2004.09.017
Ricci A, Olejar KJ, Parpinello GP, Kilmartin PA, Versari A (2015) Application of fourier transform infrared (FTIR) spectroscopy in the characterization of tannins. Appl Spectrosc Rev 50(5):407–442. https://doi.org/10.1080/05704928.2014.1000461
Andreyeva IV, Koton MM, Artem’eva VN, Sazanov YN, Fedorova GN (1976) The structure of acrolein polymers. Polym Sci USSR 18(8):1951–1959. https://doi.org/10.1016/0032-3950(76)90376-2
Li Y, Tang J, Liu Y, Li T, Ma D, Gao J, Yang J, Zhou Y, Zhang Y-F (2019) Microwave assisted polymeric modification of graphite oxide and graphite by poly(allyl diazoacetate-co-acrolein). Mater Design 183:108116. https://doi.org/10.1016/j.matdes.2019.108116
Niu S, Zhou Y, Yu H, Lu C, Han K (2017) Investigation on thermal degradation properties of oleic acid and its methyl and ethyl esters through TG-FTIR. Energy Convers Manage 149:495–504. https://doi.org/10.1016/j.enconman.2017.07.053
Sharma RK, Kumar R, Singh AP (2019) Metal ions and organic dyes sorption applications of cellulose grafted with binary vinyl monomers. Sep Purif Technol 209:684–697. https://doi.org/10.1016/j.seppur.2018.09.011
Yan X, Liu X, Qi C, Lin C, Li P, Wang H (2017) Disposal of hexabromocyclododecane (HBCD) by grinding assisted with sodium persulfate. RSC Adv 7(38):23313–23318. https://doi.org/10.1039/c7ra02689g
Acknowledgements
This work was supported by Hunan Provincial Natural Science Foundation of China (Grant No. 2019JJ50652), Scientific Research Fund of Hunan Provincial Education Department (Grant No. 18C0197) and the Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation (Grant No. 2019CL01).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, YF., Tang, J., Li, T. et al. UV-mediated atom transfer radical polymerization of acrolein. Polym. Bull. 79, 1057–1068 (2022). https://doi.org/10.1007/s00289-021-03544-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03544-w