Abstract
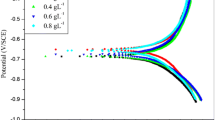
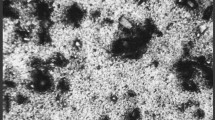
Copper is a fascinating metal with wide applications since ancient time. Although Copper is resistant to certain environmental factors and several chemicals, aggressive medium could deteriorate the metal leading to its complete damage. The need for an eco-friendly corrosion inhibitor is much relevant in the present scenario. The inhibition efficiency of gum arabic (GA), sodium alginate (SA) and its blend is investigated using weight loss method, potentiodynamic polarization and electroscopic impedance spectroscopy (EIS) analysis in 1.0 M HCl solution. Higher inhibition efficiency is obtained at room temperature for the blend with 6666 ppm GA and 3333 ppm SA. The values of inhibition efficiency obtained are 88.8%, 80.5% and 86.2% using weight loss, potentiodynamic polarization and EIS analysis, respectively. The investigations are complemented by atomic force microscopy and field emission scanning electron microscopy which helped to analyze the physical adsorption of the biopolymer on the metal surface. The adsorption of the biopolymer on Copper obeyed Langmuir isotherm and inhibition efficiency has an inverse relation with temperature.






Similar content being viewed by others
Code availability
All data are available online in Mendeley dataset through the given code: 10.17632/mj6jd2cp75.4.
References
Sherif E-SM (2012) Corrosion behavior of copper in 0.50 M hydrochloric acid pickling solutions and its inhibition by 3-amino-1, 2, 4-triazole and 3-amino-5-mercapto-1, 2, 4-triazole. Int J Electrochem Sci 7:1884–1897
Saranarayanan R, Lakshminarayanan AK, Venkatraman B (2019) A combined full-field imaging and metallography approach to assess the local properties of gas tungsten arc welded copper–stainless steel joints. Arch Civ Mech Eng 19(1):251–267
Gong W, Xu B, Yin X, Liu Y, Chen Y, Yang W (2019) Halogen-substituted thiazole derivatives as corrosion inhibitors for mild steel in 0.5 M sulfuric acid at high temperature. J Taiwan Inst Chem Eng 97:466–479
Li X, Deng S, Xie X (2014) Experimental and theoretical study on corrosion inhibition of o-phenanthroline for aluminum in HCl solution. J Taiwan Inst Chem Eng 45(4):1865–1875
Palanisamy G (2019) Corrosion inhibitors. In: Singh A (ed) Corrosion inhibitors. IntechOpen, London
Lece HD, Emregül KC, Atakol O (2008) Difference in the inhibitive effect of some Schiff base compounds containing oxygen, nitrogen and sulfur donors. Corros Sci 50(5):1460–1468
Zarras P, Stenger-Smith JD (2015) Smart inorganic and organic pretreatment coatings for the inhibition of corrosion on metals/alloys. In: Tiwari A, Rawlins J, Hihara LH (eds) Intelligent coatings for corrosion control. Elsevier, Amsterdam, pp 59–91
Swaroop BS, Victoria SN, Manivannan R (2016) Azadirachta indica leaves extract as inhibitor for microbial corrosion of copper by Arthrobacter sulfureus in neutral pH conditions—a remedy to blue green water problem. J Taiwan Inst Chem Eng 64:269–278
Kim S et al (2020) Development of polyetherimide composites for use as 3D printed thermal protection material. J Mater Sci. https://doi.org/10.1007/s10853-020-04676-6
Azzaoui K et al (2017) Eco friendly green inhibitor Gum Arabic (GA) for the corrosion control of mild steel in hydrochloric acid medium. Corros Sci 129:70–81
Dang N, Wei YH, Hou LF, Li YG, Guo CL (2015) Investigation of the inhibition effect of the environmentally friendly inhibitor sodium alginate on magnesium alloy in sodium chloride solution. Mater Corros 66(11):1354–1362
Shen C, Alvarez V, Koenig JDB, Luo J-L (2019) Gum Arabic as corrosion inhibitor in the oil industry: experimental and theoretical studies. Corros Eng Sci Technol 54(5):444–454
Umoren SA, Obot IB, Ebenso EE, Okafor PC, Ogbobe O, Oguzie EE (2006) Gum arabic as a potential corrosion inhibitor for aluminium in alkaline medium and its adsorption characteristics. Anti Corros Methods Mater 53(5):277–282
Obot IB, Onyeachu IB, Kumar AM (2017) Sodium alginate: a promising biopolymer for corrosion protection of API X60 high strength carbon steel in saline medium. Carbohydr Polym 178:200–208
Verbeken D, Dierckx S, Dewettinck K (2003) Exudate gums: occurrence, production, and applications. Appl Microbiol Biotechnol 63(1):10–21
Umoren SA (2008) Inhibition of aluminium and mild steel corrosion in acidic medium using Gum Arabic. Cellulose 15(5):751
Papageorgiou SK, Kouvelos EP, Favvas EP, Sapalidis AA, Romanos GE, Katsaros FK (2010) Metal–carboxylate interactions in metal–alginate complexes studied with FTIR spectroscopy. Carbohydr Res 345(4):469–473
Jmiai A, El Ibrahimi B, Tara A, El Issami S, Jbara O, Bazzi L (2018) Alginate biopolymer as green corrosion inhibitor for copper in 1 M hydrochloric acid: experimental and theoretical approaches. J Mol Struct 1157:408–417
Bindhu B, Nair RM (2020) A polymer blend from Gum Arabic and Sodium Alginate-preparation and characterization. J Polym Res. https://doi.org/10.1007/s10965-020-02128-y
Izadi M, Shahrabi T, Ramezanzadeh B (2017) Electrochemical investigations of the corrosion resistance of a hybrid sol–gel film containing green corrosion inhibitor-encapsulated nanocontainers. J Taiwan Inst Chem Eng 81:356–372
Sangeetha Y, Meenakshi S, Sundaram CS (2016) Corrosion inhibition of aminated hydroxyl ethyl cellulose on mild steel in acidic condition. Carbohydr Polym 150:13–20
Hamdy A, El-Gendy NS (2013) Thermodynamic, adsorption and electrochemical studies for corrosion inhibition of carbon steel by henna extract in acid medium. Egypt J Pet 22(1):17–25
Kumar S et al (2016) Experimental and theoretical studies on inhibition of mild steel corrosion by some synthesized polyurethane tri-block co-polymers. Sci Rep 6:30937
Shukla SK, Ebenso EE (2011) Corrosion inhibition, adsorption behavior and thermodynamic properties of streptomycin on mild steel in hydrochloric acid medium. Int J Electrochem Sci 6(8):3277–3291
Tawfik SM (2015) Alginate surfactant derivatives as ecofriendly corrosion inhibitor for carbon steel in acidic environment. RSC Adv 5:104535–104550
Acknowledgement
We have to express our appreciation to J Joseph (Dept. of Chemistry, Noorul Islam Centre for Higher Education) for extending his research facilities to carry out our research work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Availability of data and material
Required data or original images obtained during the study will be available from the corresponding author on request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nair, R.M., Bindhu, B., Manikandanath, N.T. et al. An eco-friendly green biopolymer blend for copper corrosion inhibition. Polym. Bull. 79, 121–136 (2022). https://doi.org/10.1007/s00289-020-03491-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03491-y