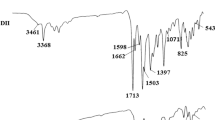
Abstract
Two new diphenol Schiff base monomers were prepared through condensation reaction between 4-hydroxybenzaldehyde and 4,4′-diaminodiphenyl sulfone (dapsone) or 1,2-cyclohexanediamine. The resulting Schiff bases were reacted with terephthaloyl dichloride through polycondensation and formed two novel poly(azomethine-ester)s. The molecular masses of diphenol Schiff bases were determined through E.I mass spectroscopy. The elemental composition of poly(azomethine-ester)s was evaluated through CHN analysis. The structures of diphenol Schiff bases and poly(azomethine-ester)s were confirmed though FT-IR, 1HNMR, UV–Vis spectroscopy, fluorescence, SEM and thermal analysis (TG/DTA). All the synthesized compounds were fluorescent and indicated 2–4 emission bands with maximum emission intensities within 342–682 nm at different excitation wavelengths. The diphenol Schiff bases showed violet-light emission, while their derived polyesters showed red-light emission. The poly(azomethine-ester)s indicated good thermal stability (347 °C and 561 °C) which was estimated through \(T_{ \hbox{max} }\) (temperature at which rate of weight loss is maximum) value obtained from DTG (derivative thermogravimetry) graph. The synthesized compounds were also tested for their antimicrobial activities against different species of bacteria and fungi. The Schiff base having dapsone moiety showed 55% inhibition, while its derived poly(azomethine-ester) showed 40% inhibition against bacterial species Shigella flexneri.









Similar content being viewed by others
References
Temizkan K, Kaya İ (2017) Synthesis, characterization, electrochemical and surface morphology properties of poly (azomethine-ester) s. Polym Bull 74:2575–2592. https://doi.org/10.1007/s00289-016-1851-8
Qureshi F, Khuhawar MY, Jahangir TM, Channar AH (2016) Synthesis, characterization and biological studies of new linear thermally stable Schiff base polymers with flexible spacers. Acta Chim Slov 63:113–120. https://doi.org/10.17344/acsi.2015.1994
Niu H, Huang Y, Bai X, Li X, Zhang G (2004) Study on crystallization, thermal stability and hole transport properties of conjugated polyazomethine materials containing 4,4′-bisamine-triphenylamine. Mater Chem Phys 86:33–37. https://doi.org/10.1016/j.matchemphys.2004.01.023
Kil Choi M, Lim Kim H, Hack Suh D (2006) Changes of fluorescence color in novel poly (azomethine) by the acidity variation. J Appl Polym Sci 101:1228–1233. https://doi.org/10.1002/app.24209
Qureshi F, Khuhawar MY, Jahangir TM (2018) Synthesis and characterization of new photoresponsive, ortho and para oriented azomethine polymers. Acta Chim Slov 65:718–729. https://doi.org/10.17344/acsi.2018.4419
Qureshi F, Khuhawar MY, Jahangir TM (2019) New fluorescent, thermally stable and film forming polyimines containing naphthyl rings. Acta Chim Slov 66:899–912. https://doi.org/10.17344/acsi.2019.5100
Baran NY, Saçak M (2018) Preparation of highly thermally stable and conductive Schiff base polymer: molecular weight monitoring and investigation of antimicrobial properties. J Mol Struct 1163:22–32. https://doi.org/10.1016/j.molstruc.2018.02.088
Qureshi F, Memon SQ, Khuhawar MY, Jahangir TM (2020) Removal of Co2+, Cu2+ and Au3+ ions from contaminated wastewater by using new fluorescent and antibacterial polymer as sorbent. Polym Bull. https://doi.org/10.1007/s00289-020-03170-y
Danjani AG, Salisu AA, Usman AH (2016) Preparation and characterization of dialdehyde 2,3-diaminopyridine starch chelating polymer and its sorption potential for Cd(II), Cu(II) and Ni(II) ions in aqueous media. BAJOPAS 9:174–178. https://doi.org/10.4314/bajopas.v9i2.32
Bhatt VD, Ray A (2001) Synthesis, characterization and electrical conductivity of polyesters containing azomethine linkages. Int J Polym Mater 49:355–366. https://doi.org/10.1080/00914030108039786
Vasanthi BJ, Ravikumar L (2013) Synthesis and characterization of poly (azomethine ester) s with a pendent dimethoxy benzylidene group. Open J Polym Chem 3:35310. https://doi.org/10.4236/ojpchem.2013.33013
Bhuvaneshwari B, Selvaraj A, Iyer NR, Ravikumar L (2015) Electrochemical investigations on the performance of newly synthesized azomethine polyester on rebar corrosion. Mater Corros 66:387–395. https://doi.org/10.1002/maco.201307472
Temizkan K, Kaya İ (2017) Heat resisting and water-soluble chocolate polyesters containing azomethine group. Mater Sci Pol 35:303–312. https://doi.org/10.1515/msp-2017-0018
Zhao D, Wang J, Wang X-L, Wang Y-Z (2018) Highly thermostable and durably flame-retardant unsaturated polyester modified by a novel polymeric flame retardant containing Schiff base and spirocyclic structures. Chem Eng J 344:419–430. https://doi.org/10.1016/j.cej.2018.03.102
Wu J-N, Chen L, Fu T, Zhao H-B, Guo D-M, Wang X-L, Wang Y-Z (2018) New application for aromatic Schiff base: high efficient flame-retardant and anti-dripping action for polyesters. Chem Eng J 336:622–632. https://doi.org/10.1016/j.cej.2017.12.047
Iwan A, Palewicz M, Sikora A, Chmielowiec J, Hreniak A, Pasciak G, Bilski P (2010) Aliphatic–aromatic poly (azomethine) s with ester groups as thermotropic materials for opto (electronic) applications. Synth Met 160:1856–1867. https://doi.org/10.1016/j.synthmet.2010.06.029
Nishat N, Parveen S, Dhyani S, Asma (2009) Antimicrobial polyesters containing Schiff-base metal complexes. J Coord Chem 62:1091–1099. https://doi.org/10.1080/00958970802416020
Lim W-L, Oo C-W, Choo Y-S-L, Looi S-T (2015) New generation of photosensitive poly (azomethine) esters: thermal behaviours, photocrosslinking and photoluminescence studies. Polymer 71:15–22. https://doi.org/10.1016/j.polymer.2015.06.041
Sanadhya SG, Oswal SL, Parmar KC (2014) Synthesis and characterization of wholly aromatic polyesters using interfacial polycondensation technique. Der Pharm Chem 6:156–163
Vini R, Murugavel SC (2020) Photoisomerization and thermal degradation kinetics of poly (ether–ester)s containing azomethine moiety in the main chain. High Perform Polym 32:47–58. https://doi.org/10.1177/0954008319850614
Gong J, Yakushi Y, Uchida T, Yamazaki S, Kimura K (2011) One-pot preparation of aromatic poly (azomethine ester) fibrillar crystals using reaction-Induced crystallization. J Polym Sci A Polym Chem 49:127–137. https://doi.org/10.1002/pola.24427
Şenol D, Kaya İ (2017) Synthesis and characterization of azomethine polymers containing ether and ester groups. J Saudi Chem Soc 21:505–516. https://doi.org/10.1016/j.jscs.2015.05.006
Thomas O, Inganäs O, Andersson MR (1998) Synthesis and properties of a soluble conjugated poly (azomethine) with high molecular weight. Macromolecules 31:2676–2678. https://doi.org/10.1021/ma9701090
Yi MH, Huang W, Jin MY, Choi K-Y (1997) Synthesis and characterization of soluble polyimides from 1,1-bis (4-aminophenyl) cyclohexane derivatives. Macromolecules 30:5606–5611. https://doi.org/10.1021/ma9703665
Choi KY, Yi MH (1999) Soluble polyimides containing alicyclic structures. Macromol Symp 142:193–204. https://doi.org/10.1002/masy.19991420119
Liaw D-J (1995) Synthesis and characterization of sulfone-containing polyesters derived from 4,4′-dicarboxydiphenyl sulfone by direct polycondensation. J Polym Sci A Polym Chem 33:605–613. https://doi.org/10.1002/pola.1995.080330401
Mehdipour-Ataei S, Sarrafi Y, Hatami M, Akbarian-Feizi L (2005) Poly (sulfone ether amide amide) s as a new generation of soluble, thermally stable polymers. Eur Polym J 41:491–499. https://doi.org/10.1016/j.eurpolymj.2004.10.009
Pettit R-K, Weber C-A, Kean M-J, Hoffmann H, Pettit G-R, Tan R, Franks K-S, Horton M-L (2005) Microplate Alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob Agents Chemother 49:2612–2617. https://doi.org/10.1128/aac.49.7.2612-2617.2005
Bhatt VD, Ray A (1998) Synthesis, characterization and electrical conductivity of polyesters, polyamides and doped polymers. Synth Met 92:115–120. https://doi.org/10.1016/S0379-6779(98)80100-8
Channar AH, Sheikh AA, Khuhawar MY, Majidano SA, Pirzada T (2013) Preparation and characterization of new polyesters derived from schiff bases. J Chem Soc Pak 35:151–156
Kaya İ, Kılavuz E (2015) Novel multicolor Schiff base polymers prepared via oxidative polycondensation. J Fluoresc 25:663–673. https://doi.org/10.1007/s10895-015-1552-y
Kaya İ, Avcı A, Temizkan K (2017) Photophysical and thermal properties of polyazomethines containing various flexible units. Macromol Res 25:45–53. https://doi.org/10.1007/s13233-017-5002-3
Mighani H, Fathollahi E, Ghaemy M (2015) An alternative method for synthesis of thermally stable aromatic polyesters containing Schiff base unites. JACR 9:103–109
Kaya İ, Kartal E, Şenol D (2015) Synthesis and characterization of polyphenol derived from Schiff bases containing methyl and carboxyl groups in the structure. Des Monomers Polym 18:524–535. https://doi.org/10.1080/15685551.2015.1041084
Choi YC, Kim MS, Ryu KM, Lee SH, Jeong YG (2020) Synthesis and characterization of aromatic poly (azomethine ether) s with different meta-and para-phenylene linkage contents. Fibers Polym 21:238–244. https://doi.org/10.1007/s12221-020-9690-5
AZoNano (2009) Coventry still makes transport; unique molecular delivery systems for functional polymers. https://www.azonano.com/article.aspx?ArticleID=2409
Courgneau C, Rusu D, Henneuse C, Ducruet V, Lacrampe M, Krawczak P (2013) Characterisation of low-odour emissive polylactide/cellulose fibre biocomposites for car interior. Express Polym Lett 7:787–804. https://doi.org/10.3144/expresspolymlett.2013.76
Lynch DE, Nawaz Y, Bostrom T (2005) Preparation of sub-micrometer silica shells using poly (1-methylpyrrol-2-ylsquaraine). Langmuir 21:6572–6575. https://doi.org/10.1021/la0506933
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qureshi, F., Khuhawar, M.Y., Jahangir, T.M. et al. Synthesis and characterization of new thermally stable, antimicrobial and red-light-emitting poly(azomethine-ester)s. Polym. Bull. 78, 5055–5074 (2021). https://doi.org/10.1007/s00289-020-03357-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03357-3