Abstract
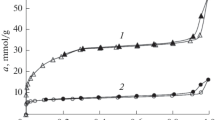
Metal–organic frameworks (MOFs) are presented as potential candidates to meet the challenge of storing hydrogen at room temperature. However, few hydrogen adsorbents balance high volumetric and gravimetric capacities. In this work, a series of ten MOFs of different topologies were selected to determine on the one hand the characteristics of MOFs capable of balancing the volumetric and gravimetric adsorption capacities of hydrogen at room temperature and on the other hand a correlation between the adsorption properties and the structure of MOF. The influence of characteristics such as free volume, pore diameter, and isosteric heat of adsorption on optimal adsorption at room temperature was evaluated. The results showed that the absolute gravimetric absorption capacity is well correlated with the free volume of the pores. The gravimetric and volumetric capacities of hydrogen absorbents can be balanced and increased by using materials that provide high isosteric heat of absorption. Small pore materials provide high isosteric adsorption heat but adsorb less due to the low free volume.









Similar content being viewed by others
References
Bercegol Hervé, Didierjean Sophie, Étienne Mathieu et al., « De nouvelles technologies de l’énergie en rupture ? », Annales des Mines–Responsabilité et environnement, 2019/3 (N° 95), p. 62–66. https://www.cairn.info/revue-responsabilite-et-environnement-2019–3-page-62.htm. Accessed 20 Nov 2019
Basdogan Y, Keskin S (2015) Simulation and modelling of MOFs for hydrogen storage. CrystEngComm 17(2):261–275. https://doi.org/10.1039/c4ce01711k
Prachi Prabhukhot R, Wagh MM, Gangal Aneesh C (2016) A review on solid state hydrogen storage material. Adv Energy Power 4(2):11–22. https://doi.org/10.13189/aep.2016.040202
Li H, Wang K, Sun Y, Lollar CT, Li J, Zhou H-C (2018) Recent advances in gas storage and separation using metal–organic frameworks. Mater Today 21(2):108–121. https://doi.org/10.1016/j.mattod.2017.07.006
Ghiyasiyan-Arani M, Salavati-Niasari M (2018) Effect of Li2CoMn3O8 Nanostructures synthesized by a combustion method on montmorillonite K10 as a potential hydrogen storage material. J Phys Chem C 122(29):16498–16509. https://doi.org/10.1021/acs.jpcc.8b02617
Zohuri B (2018) Hydrogen: driving renewable energy. Hydrogen Energy. https://doi.org/10.1007/978-3-319-93461-7_5
Brangier E, Vivian R, Bornet C (2019) Méthodes d’ergonomie prospective appliquées à l’identification de besoins pour des systèmes d’énergie à base d’hydrogène : étude exploratoire. Psychol Française. https://doi.org/10.1016/j.psfr.2019.02.002
García-Holley P, Schweitzer B, Islamoglu T, Liu Y, Lin L, Rodriguez S, Farha OK (2018) Benchmark study of hydrogen storage in metal-organic frameworks under temperature and pressure swing conditions. ACS Energy Letters 3(3):748–754. https://doi.org/10.1021/acsenergylett.8b00154
Kothari R, Buddhi D, Sawhney RL (2008) Comparison of environmental and economic aspects of various hydrogen production methods. Renew Sustain Energy Rev 12(2):553–563. https://doi.org/10.1016/j.rser.2006.07
A. Kamaruddin, G. Postole, A. Auroux, B. Galey. Etude de l’impact de la nanostructuration de MgH2 sur les propriétés de stockage de l’hydrogène. Journée de Printemps de la section Rhône-Alpes de la SCF, Jun 2019, Saint-Martin d'Hères, France. ⟨hal-02174523⟩
Menia S, Nouicer I, Bakouri Y, M’raoui A, Tebibel H, Khellaf A (2019) Production d’hydrogène par procédés biologiques. Oil Gas Sci Technol Rev IFP Energ Nouvelles 74:34. https://doi.org/10.2516/ogst/2018099
Gholami T, Salavati-Niasari M, Salehabadi A, Amiri M, Shabani-Nooshabadi M, Rezaie M (2018) Electrochemical hydrogen storage properties of NiAl 2 O 4 /NiO nanostructures using TiO 2, SiO 2 and graphene by auto-combustion method using green tea extract. Renew Energy 115:199–207. https://doi.org/10.1016/j.renene.2017.08.037
Jepsen J, Capurso G, Puszkiel J, Busch N, Werner T, Milanese C, Klassen T (2019) Effect of the process parameters on the energy transfer during the synthesis of the 2LiBH4-MgH2 reactive hydride composite for hydrogen storage. Metals 9(3):349. https://doi.org/10.3390/met9030349
Bossel, U.; Eliasson, B. Energy and the Hydrogen Economy, US DOE, EERE. Available online: https://www.afdc.energy.gov/pdfs/hyd_economy_bossel_eliasson.pdf (accessed on 15 February 2019).
Von Helmolt R, Eberle U (2007) Fuel cell vehicles: status 2007. J Power Sources 165(2):833–843. https://doi.org/10.1016/j.jpowsour.2006.12.073
Jepsen J, Bellosta von Colbe JM, Klassen T, Dornheim M (2012) Economic potential of complex hydrides compared to conventional hydrogen storage systems. Int J Hydrogen Energy 37(5):4204–4214. https://doi.org/10.1016/j.ijhydene.2011.11.141
Purewal J, Veenstra M, Tamburello D, Ahmed A, Matzger AJ, Wong-Foy AG, Siegel DJ (2019) Estimation of system-level hydrogen storage for metal-organic frameworks with high volumetric storage density. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2019.04.082
Demirocak DE, Srinivasan SS, Ram MK, Goswami DY, Stefanakos EK (2013) Volumetric hydrogen sorption measurements—Uncertainty error analysis and the importance of thermal equilibration time. Int J Hydrogen Energy 38(3):1469–1477. https://doi.org/10.1016/j.ijhydene.2012.11.013
Suh MP, Park HJ, Prasad TK, Lim D-W (2011) Hydrogen storage in metal-organic frameworks. Chem Rev 112(2):782–835. https://doi.org/10.1021/cr200274s
Thornton, Matthew J., and Simpson, Lin J. System Design, Analysis, and Modeling Activities Supporting the DOE Hydrogen Storage Engineering Center of Excellence (HSECoE): Final Project Report. United States: N. p., 2019. Web. https://doi.org/10.2172/1507683
Camp J, Stavila V, Allendorf MD, Prendergast D, Haranczyk M (2018) Critical factors in computational characterization of hydrogen storage in metal-organic frameworks. J Phys Chem C 122(33):18957–18967. https://doi.org/10.1021/acs.jpcc.8b04021
Hirscher M, Panella B, Schmitz B (2010) Metal-organic frameworks for hydrogen storage. Microporous Mesoporous Mater 129(3):335–339. https://doi.org/10.1016/j.micromeso.2009.06.005
Nijkamp MG, Raaymakers JEMJ, van Dillen AJ, de Jong KP (2001) Hydrogen storage using physisorption—materials demands. Appl Phys A Mater Sci Process 72(5):619–623. https://doi.org/10.1007/s003390100847
Frost H, Snurr RQ (2007) Design requirements for metal-organic frameworks as hydrogen storage materials. J Phys Chem C 111(50):18794–18803. https://doi.org/10.1021/jp076657p
Bhatia SK, Myers AL (2006) Optimum conditions for adsorptive storage. Langmuir 22(4):1688–1700. https://doi.org/10.1021/la0523816
Thornton AW, Simon CM, Kim J, Kwon O, Deeg KS, Konstas K, Smit B (2017) Materials genome in action: identifying the performance limits of physical hydrogen storage. Chem Mater 29(7):2844–2854. https://doi.org/10.1021/acs.chemmater.6b04933
Anton DL, Motyka T (2015) Hydrogen storage engineering center of excellence. United States Department of Energy, USA
Veenstra, M., et al. Ford/BASF-SE/UM Activities in Support of the Hydrogen Storage Engineering Center of Excellence. https://www.hydrogen.energy.gov/pdfs/review14/st010_veenstra_2014_o.pdf (United States Department of Energy, Hydrogen and Fuel Cells Program 2014 Annual Merit Review Proceedings: Project ST010, USA, 2014). Accessed 20 Nov 2019
Ahmed A, Seth S, Purewal J, Wong-Foy AG, Veenstra M, Matzger AJ, Siegel DJ (2019) Exceptional hydrogen storage achieved by screening nearly half a million metal-organic frameworks. Nat Commun. https://doi.org/10.1038/s41467-019-09365w
Ahmed A, Liu Y, Purewal J, Tran LD, Wong-Foy AG, Veenstra M, Siegel DJ (2017) Balancing gravimetric and volumetric hydrogen density in MOFs. Energy Environ Sci 10(11):2459–2471. https://doi.org/10.1039/c7ee02477k
Yuan D, Zhao D, Sun D, Zhou H-C (2010) An isoreticular series of metal-organic frameworks with dendritic hexacarboxylate ligands and exceptionally high gas-uptake capacity. Angew Chem Int Ed 49(31):5357–5361. https://doi.org/10.1002/anie.201001009
Prasad TK, Suh MP (2012) Control of interpenetration and gas-sorption properties of metal-organic frameworks by a simple change in ligand design. Chem Eur J 18(28):8673–8680. https://doi.org/10.1002/chem.201200456
Ahmed A, Hodgson N, Barrow M, Clowes R, Robertson CM, Steiner A, Zhang H (2014) Macroporous metal–organic framework microparticles with improved liquid phase separation. J Mater Chem A 2(24):9085–9090. https://doi.org/10.1039/c4ta00138a
Feng Y, Wang T, Li Y, Li J, Wu J, Wu B, Wang C (2015) Steering metallofullerene electron spin in porous metal-organic framework. J Am Chem Soc 137(47):15055–15060. https://doi.org/10.1021/jacs.5b10796
Wong-Ng W, Kaduk JA, Wu H, Suchomel M (2012) Synchrotron X-ray studies of metal-organic framework M 2(2,5-dihydroxyterephthalate), M = (Mn Co, Ni, Zn) (MOF74). Powder Diffr 27(04):256–262. https://doi.org/10.1017/s0885715612000863
Getman RB, Bae Y-S, Wilmer CE, Snurr RQ (2011) Review and analysis of molecular simulations of methane, hydrogen, and acetylene storage in metal-organic frameworks. Chem Rev 112(2):703–723. https://doi.org/10.1021/cr200217c
Darkrim F, Levesque D (1998) Monte Carlo simulations of hydrogen adsorption in single-walled carbon nanotubes. J Chem Phys 109(12):4981–4984. https://doi.org/10.1063/1.477109
Rappe AK, Casewit CJ, Colwell KS, Goddard WA, Skiff WM (1992) UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J Am Chem Soc 114(25):10024–10035. https://doi.org/10.1021/ja00051a040
Mayo SL, Olafson BD, Goddard WA (1990) DREIDING: a generic force field for molecular simulations. J Phys Chem 94(26):8897–8909. https://doi.org/10.1021/j100389a010
Boda D, Henderson D (2008) The effects of deviations from Lorentz-Berthelot rules on the properties of a simple mixture. Mol Phys 106(20):2367–2370. https://doi.org/10.1080/00268970802471137
Dubbeldam D, Calero S, Ellis DE, Snurr RQ (2016) RASPA: molecular simulation software for adsorption and diffusion in flexible nanoporous materials. Mol Simul 42(2):81–101. https://doi.org/10.1080/08927022.2015.1010082
Martin RL, Haranczyk M (2014) Construction and characterization of structure models of crystalline porous polymers. Cryst Growth Des 14(5):2431–2440. https://doi.org/10.1021/cg500158c
Panella B, Hirscher M, Roth S (2005) Hydrogen adsorption in different carbon nanostructures. Carbon 43:2209–2214
Gómez-Gualdrón DA, Wang TC, García-Holley P, Sawelewa RM, Argueta E, Snurr RQ, Farha OK (2017) Understanding volumetric and gravimetric hydrogen adsorption trade-off in metal-organic frameworks. ACS Appl Mater Interfaces 9(39):33419–33428. https://doi.org/10.1021/acsami.7b01190
Acknowledgements
The authors are thankful to The Cambridge Crystallographic Data Center (CCDC) for the material files graciously made available to us through Dr Yves Alain Mbiangué and to the developers of the RASPA particularly Dr Randall Snurr and Zeo ++ software with which we made simulations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest in regard with this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Assoualaye, G., Djongyang, N. Influence of pore size and isosteric heat of adsorption of some metal–organic frameworks on the volumetric and gravimetric adsorption capacities of hydrogen at room temperature. Polym. Bull. 78, 4987–5001 (2021). https://doi.org/10.1007/s00289-020-03350-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03350-w