Abstract
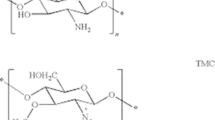
Conjugation of insulin-mimetic [meso-tetrakis(4-sulfonatophenyl)porphyrinato]Zn(II), Zn(tpps), with chitosan was examined in aqueous solution at various contact time, Zn(tpps) concentrations and solution temperatures, respectively. The chitosan–Zn(tpps) conjugate was characterized by UV–Vis and Fourier transform infrared (FTIR) spectroscopic techniques. The electrostatic interaction between negatively charged sulfonate \(({\mathrm{SO}}_{3}^{-})\) groups of Zn(tpps) and positively charged amino (\({\mathrm{NH}}_{3}^{+}\)) groups of chitosan is responsible for the formation of chitosan–Zn(tpps) conjugate. Chitosan efficiently inhibits the demetallization and aggregation of Zn(tpps) in acidic aqueous solution. The conjugation kinetic data taken from various batch studies were examined by the pseudo-first-order, pseudo-second-order, Elovich kinetic, film diffusion and intra-particle diffusion models. The equilibrium conjugation isotherms were analyzed by Freundlich, Temkin and Langmuir isotherm models, respectively. The batch conjugation kinetic data were obeyed by the pseudo-second-order kinetic model rather than the pseudo-first-order and Elovich kinetic models. Equilibrium conjugation isotherms were explained well by the Langmuir isotherm model, and the highest extent of Zn(tpps) bound to chitosan was obtained to be 151.52 µmol/g at 45 °C. The activation energy (Ea: 10.21 kJ/mol), changes in Gibb’s free energy (∆G: –26.38 to –28.12 kJ/mol), enthalpy (∆H: 8.74 kJ/mol) and entropy (∆S: 115.94 J/mol K) suggest that the chitosan–Zn(tpps) conjugation is an endothermic spontaneous process. The in vitro release of Zn(tpps) from chitosan–Zn(tpps) conjugate was investigated in phosphate buffer solution (pH 7.4) at 37 °C. The kinetics of Zn(tpps) release followed the first-order kinetics with a rate constant 0.0025 h‒1. The chitosan–Zn(tpps) conjugate may be used as an oral therapeutics for diabetes mellitus.










taken from b); and (d) a plot log [residual Zn(tpps) (%)] versus time (data were taken from b)
Similar content being viewed by others
References
Torres FG, Troncoso OP, Pisani A, Gatto F, Bardi G (2019) Natural polysaccharide nanomaterials: an overview of their immunological properties. Int J Mol Sci 20:5092–5113
Komi DEA, Hamblin MR (2016) Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int J Adv Res (Indore) 4:411–427
Xiao F, Cheng J, Cao W, Yang C, Chen J, Luo Z (2019) Removal of heavy metals from aqueous solution using chitosan-combined magnetic biochars. J Colloid Interface Sci 540:579–584
Ali I (2012) New generation adsorbents for water treatment. Chem Rev 112:5073–5091
Karmaker S, Nag AJ, Saha TK (2019) Adsorption of remazol brilliant violet onto chitosan 10B in aqueous solution: kinetics, equilibrium and thermodynamics studies. Cellulose Chem Technol 53:373–386
Saha TK, Karmaker S, Ichikawa H, Fukumori Y (2005) Mechanisms and kinetics of trisodium 2-hydroxy-1,1′-azonaphthalene-3,4′,6-trisulfonate adsorption onto chitosan. J Colloid Interface Sci 286:433–439
Karmaker S, Sen T, Saha TK (2015) Adsorption of reactive yellow 145 onto chitosan in aqueous solution: kinetic modeling and thermodynamic analysis. Polym Bull 72:1879–1897
Karmaker S, Sintaha F, Saha TK (2019) Kinetics, isotherm and thermodynamic studies of the adsorption of reactive red 239 dye from aqueous solution by chitosan 8B. Adv Biol Chem 9:1–22
Imran M, Ramzan M, Qureshi AK, Khan MA, Tariq M (2018) Emerging applications of porphyrins and metalloporphyrins in biomedicine and diagnostic magnetic resonance imaging. Biosensors 8:95–111
Ptaszynska AA, Trytek M, Borsuk G, Buczek K, Rybicka-Jasinska K, Gryko D (2018) Porphyrins inactivate Nosema spp. microsporidia. Sci Rep 8:5523
Varchi G, Foglietta F, Canaparo R, Ballestri M, Arena F, Sotgiu G, Fanti S (2015) Engineered porphyrin loaded core–SHELL nanoparticles for selective sonodynamic anticancer treatment. Nanomedicine 10:3483–3494
Saha TK, Frauendorf H, John M, Dechert S, Meyer F (2013) Efficient oxidative degradation of azo dyes by a water-soluble manganese porphyrin catalyst. ChemCatChem 5:796–805
Huang H, Song W, Rieffel J, Lovell JF (2015) Emerging applications of porphyrins in photomedicine. Fron Phys 3:1–23
Cheng W, Haedicke IE, Nofiele J, Martinez F, Beera K, Scholl TJ, Zhang XA (2014) Complementary strategies for developing Gd-free high-field T1 MRI contrast agents based on MnIII porphyrins. J Med Chem 57:516–520
Hammerer F, Garcia G, Chen S, Poyer F, Achelle S, Fiorini-Debuisschert C, Maillard P (2014) Synthesis and characterization of glycoconjugated porphyrin triphenylamine hybrids for targeted two-photon photodynamic therapy. J Org Chem 79:1406–1417
Dong X, Chen H, Qin J, Wei C, Liang J, Liu T, Lv F (2017) Thermosensitive porphyrin-incorporated hydrogel with four-arm PEG-PCL copolymer (II): doxorubicin loaded hydrogel as a dual fluorescent drug delivery system for simultaneous imaging tracking in vivo. Drug Deliv 24:641–650
Stender AS, Marchuk K, Liu C, Sander S, Meyer MW, Smith EA, Huang B (2013) Single cell optical imaging and spectroscopy. Chem Rev 113:2469–2527
Saha TK, Yoshikawa Y, Sakurai H (2007) A [meso-Tetrakis(4-sulfonatophenyl)porphy-rinato] zinc(II) complex as an oral therapeutic for the treatment of type 2 diabetic KKAy mice. ChemMedChem 2:218–225
Chytil P, Koziolová E, Etrych T, Ulbrich K (2018) HPMA copolymer-drug conjugates with controlled tumor-specific drug release. Macromol Biosci 18:1700209
Minrath I, Arbeiter D, Schmitz KP, Sternberg K, Petersen S (2014) In vitro characterization of polyacrylamide hydrogels for application as implant coating for stimulus-responsive local drug delivery. Polym Adv Technol 25:1234–1241
Kim W, Yang Y, Kim D, Jeong S, Yoo J, Yoon JH, Jung Y (2017) Conjugation of metronidazole with dextran: a potential pharmaceutical strategy to control colonic distribution of the anti-amebic drug susceptible to metabolism by colonic microbes. Drug Des Devel Ther 11:419–429
Kumar TMP, Umesh HM, Shivakumar HG, Ravi V, Siddaramaiah (2007) Feasibility of polyvinyl alcohol as a transdermal drug delivery system for terbutaline sulphate. J Macromol Sci A 44:583–589
Karmaker S, Saha TK, Yoshikawa Y, Yasui H, Sakurai H (2006) A novel drug delivery system for type 1 diabetes: insulin-mimetic vanadyl-poly(c-glutamic acid) complex. J Inorg Biochem 100:1535–1546
Saha TK, Ichikawa H, Fukumori Y (2006) Gadolinium diethylenetriaminopetaaceitic acid-loaded chitosan microspheres for gadolinium neutron-capture therapy. Carbohydr Res 341:2835–2841
Yu X, Wen T, Cao P, Shan L, Li L (2019) Alginate-chitosan coated layered double hydroxide nanocomposites for enhanced oral vaccine delivery. J Colloid Interface Sci 556:258–265
Schnürch AB, Dünnhaupt S (2012) Chitosan-based drug delivery systems. Eur J Pharm Biopharm 81:463–469
Lee E, Lee J, Jon S (2010) A novel approach to oral delivery of insulin by conjugating with low molecular weight chitosan. Bioconjug Chem 21:1720–1723
El-Refaey A, Shaban SY, El-Kemary M, El-Khouly M (2017) Spectroscopic and thermodynamic studies of light harvesting perylenediimide derivative-zinc porphyrin complex in aqueous media. Spectrochim Acta Part A 186:132–139
Shanmugam S, Xu J, Boyer C (2016) A logic gate for external regulation of photopolymerization. Polym Chem 7:6437–6449
Synytsya A, Synytsya A, Blafkova P, Ederova J, Spevacek J, Slepicka P, Kral V, Volka K (2009) pH-controlled self-assembling of meso-tetrakis(4-sulfonatophenyl)porphyrin-chitosan complexes. Biomacromol 10:1067–1076
Venkatesan J, Jayakumar R, Mohandas A, Bhatnagar I, Kim S-K (2014) Antimicrobial activity of chitosan-carbon nanotube hydrogels. Materials 7:3946–3955
Zhang Y-H, Chen D-M, He T, Liu F-C (2003) Raman and infrared spectral study of meso-sulfonatophenyl substituted porphyrins (TPPSn, n = 1, 2A, 2O, 4, 4). Spectrochim Acta Part A 59:87–101
Brijmohan SB, Swier S, Weiss RA, Shaw MT (2005) Synthesis and characterization of cross-linked sulfonated polystyrene nanoparticles. Ind Eng Chem Res 44:8039–8045
Tesolekile N, Ncapayi V, Obiyenwa GK, Matoetoe M, Songca S, Oluwafemi OS (2019) Synthesis of meso-tetra-(4-sulfonatophenyl)porphyrin (TPPS4)-CuInS/ZnS quantum dots conjugate as an improved photosensitizer. Int J Nanomed 14:7065–7078
Kumar S, de Silva JA, Wani MY, Gil JM, Sobral AJFN (2017) Carbon dioxide capture and conversion by an environmentally friendly chitosan based meso-tetrakis(4-sulfonatophenyl) porphyrin. Carbohydr Polym 175:575–583
Wang X, Zhao L, Ma R, An Y, Shi L (2010) Stability enhancement of ZnTPPS in acidic aqueous solutions by polymeric micelles. Chem Commun 46:6560–6562
Akter B, Khan AI, Karmaker S, Ghosh P, Saha S, Polash SA, Islam Z, Sarker SR, Hossain MS, Yasui H, Saha TK (2020) Chelation of zinc(II) with poly(γ-glutamic acid) in aqueous solution: kinetics, binding constant and its antimicrobial activity. Polym Bull. https://doi.org/10.1007/s00289-020-03165-9
Saha TK, Karmaker S, Alam MF (2014) Kinetics, mechanism and thermodynamics involved in sorption of meso-tetrakis(4-sulfonatophenyl)-porphyrin onto chitosan in aqueous medium. J Porphyr Phthalocya 8:240–250
Saha TK, Bhoumik NC, Karmaker S, Ahmed MG, Ichikawa H, Fukumori Y (2011) Adsorption characteristics of reactive black 5 from aqueous solution onto chitosan. Clean Soil Air Water 39:984–993
Vijayakumar G, Dharmendrirakumar M, Renganathan S, Sivanesan S, Baskar G, Elango KP (2009) Removal of congo red from aqueous solutions by perlite. Clean Soil Air Water 37:355–364
Chiou MS, Li HY (2003) Adsorption behavior of reactive dye in aqueous Ssolution on chemical cross-linked chitosan beads. Chemosphere 50:1095–1105
Kumar MNVR (2000) A review of chitin and chitosan applications. React Funct Polym 46:1–27
Lagergren S (1898) Zur theorie der sogenannten adsorption gelöster stoffe. K Sven Vetenskapsakad Handl 24:1–39
McKay G, Ho YS (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–456
Elovich SY, Larinov OG (1962) Theory of adsorption from solutions of nonelectrolytes on solid (I) equation adsorption from solutions and the analysis of its simplest form, (II) verification of the equation of adsorption isotherm from solutions. Izv Akad Nauk SSSR Otd Khim Nauk 2:209–216
Onal Y, Basar CA, Ozdemir CS (2007) Investigation kinetics mechanisms of adsorption malachite green onto activated carbon. J Hazard Mater 146:194–203
WeberMorris WJJRJC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Proc Am Soc Civil Eng 89:31–59
Pan M, Lin X, Xie J, Huang X (2017) Kinetic, equilibrium and thermodynamic studies for phosphate adsorption on aluminum hydroxide modified polygorskite nano-composites. RSC Adv 7:4492–4500
Cheng C, Deng J, Lei B, He A, Zhang X, Ma L, Li S, Zhao C (2013) Toward 3D grapheme oxide gels based adsorbents for high-efficient water treatment via the promotion of biopolymers. J Hazard Mater 263:467–478
Freundlich H (1906) Adsorption in solutions. Z Phys Chemei 57:384–470
Temkin MI, Pyzhev V (1940) Kinetic of ammonia synthesis on promoted iron catalyst. Acta Physiochim URSS 12:327–356
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Petrolekas PD, Maggenakis G (2007) Kinetic studies of the liquid-phase adsorption of a reactive dye onto activated lignite. Ind Eng Chem Res 46:1326–1332
Saha TK, Mahmud MF, Karmaker S, Sen T (2012) Adsorption of [meso-tetrakis(4-sulfonatophenyl)porphyrinato]oxovanadate(IV) (4–) onto chitosan in aqueous solution. Polym Bull 68:1483–1500
Gupta VK, Srivastava SK, Mohan D (1997) Equilibrium uptake, Sorption dynamics, process optimization, and column operations for the removal and recovery of malachite green from wastewater using activated carbon and activated slag. Ind Eng Chem Res 36:2207–2218
Acknowledgements
The authors have declared no conflict of interest. We are indebted to the Ministry of Education, Government of the People’s Republic of Bangladesh, for giving research Grants (FY 2015-2016, 2016-2017 and 2017-2018) to Prof. Dr. Tapan Kumar Saha to complete the present work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pervin, S., Shaha, C.K., Karmaker, S. et al. Conjugation of insulin-mimetic [meso-tetrakis(4-sulfonatophenyl)porphyrinato]zinc(II) with chitosan in aqueous solution: kinetics, equilibrium and thermodynamics. Polym. Bull. 78, 4527–4550 (2021). https://doi.org/10.1007/s00289-020-03331-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03331-z