Abstract
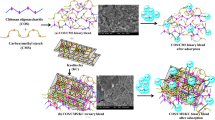
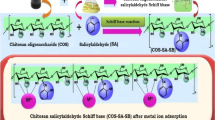
The present study was proposed to clear toxic heavy metal cadmium using a novel hybrid material of chitosan oligosaccharide-g-glycidyl methacrylate/polypropylene glycol-glutaraldehyde blend. The blend was physicochemically characterized by FTIR, XRD, and SEM analysis. The sorption capacity was brought under batch mode to suit the optimal parameters, viz. initial concentration, adsorbent dose, pH, and agitation time, which influence the sorption. Experimental data were analyzed by Langmuir and Freundlich adsorption isotherms. The isotherm study revealed that the equilibrium adsorption is well fitted to the Freundlich isotherm. The kinetic studies showed that the adsorption process follows pseudo-second-order kinetics. The adsorbed sample was characterized by SEM-EDAX and FTIR analysis, which confirmed that adsorption took place effectively. The metal-loaded sorbent was taken for desorption studies revealed that 0.1 M HCl was the best eluent for desorption of cadmium metal ions in the desorption studies. The prepared blend is active against both the selected bacterial and fungal species, and hence, it can be considered as good antimicrobial agents, and it will effectively kills the microbes. From the results, it was concluded that COS-g-GMA/PPG-Glu is an excellent material as a biosorbent for removal Cd(II) ions from aqueous solution as well as good antimicrobial activity.



















Similar content being viewed by others
References
Qaiser S, Saleemi AR, Ahmad MM (2007) Heavy metal uptake by agro based waste materials. Environ Biotechnol 10:409–416
Wang J, Chen C (2006) Biosorption of heavy metals. Biotechnol Prog 1:235–250
Regine HSF, Volesky B (2000) Biosorption: a solution to pollution. Int Microbiol 3:17–24
Dowlatshahi S, Torbati ARH, Loloei M (2014) Adsorption of copper, lead and cadmium from aqueous solutions by activated carbon prepared from saffron leaves. J Environ Health Sci 1(1):37–44
Kurniawan TA, Chan GYS, Lo WH, Babel S (2006) Physico–chemical treatment techniques for wastewater laden with heavy metals. J Chem Eng 118:83–98
Kratchovil D, Volesky B (1998) Advances in the biosorption of heavy metals. TIBTECH 16:291–300
Kuroiwa T, Noguchi Y, Nakajima M, Sato S, Mukataka S, Ichikawa S (2008) Production of chitosan oligosaccharides using chitosanase immobilized on amylose-coated magnetic nanoparticles. Process Biochem 43:62–69
Fernandez-de Castro L, Mengíbar M, Sanchez A, Arroyo L, Villaran MC, de Apodaca ED, Heras A (2016) Films of chitosan and chitosan-oligosaccharide neutralized and thermally treated: effects on its antibacterial and other activities. LWT Food Sci Technol 73:368–374
Li F, Li S, Jiang T, Sun Y (2011) Syntheses and characterization of chitosan oligosaccharide-graft-polycaprolactone copolymer I thermal and spherulite morphology studies. Adv Mater Res 83:155–160
Vismaraa E, Melonea Gastaldi L (2009) Surface functionalization of cotton cellulose with glycidyl methacrylate and its application for the adsorption of aromatic pollutant form waste water. J Hazard Mater 70:798–808
Sun T, Xie W, Xu P (2004) Superoxide anion scavenging activity of graft chitosan derivatives. Carbohydr Polym 58(4):379–382
Paul DR Sperling LH (1986) Polymer blends: phase behavior and property relationships. In: Advances in chemistry series 211 Multicomponent polymer materials. American Chemistry Society Washington, pp 3–19
Mirzaei BE, Ramazani SA, Shafiee M, Danaei M (2012) Studies on glutaraldehyde crosslinked chitosan hydrogel properties for drug delivery systems. Int J Polym Mater Polym 62:605–611
Thenmozhi N, Gomathi T, Sudha PN (2013) Preparation and characterization of biocomposites: chitosan and silk fibroin. Der Pharmacia Lett 5:88–97
Rakkapao N, Vao-soongnern V, Masubuchi Y, Watanabe H (2011) Miscibility of chitosan/poly (ethylene oxide) blends and effect of doping alkali and alkali earth metal ions on chitosan/PEO interaction. Polymer 52:2618–2627
Shukla SR, Athalye AR (1994) Graft-copolymerization of glycidyl methacrylate onto cotton cellulose. J Appl Polym Sci 54:279–285
Roller S (2003) Chitosan: new food preservative or laboratory curiosity. Woodhead Publishing, Sawston, pp 75–158
Mohammad O, Tuhin NR (2012) Modification of mechanical and thermal properties of chitosan starch blend films. Radiat Phys Chem 88:1659–1668
Ahmad N, Ahmad R, Al-Qudaihi A, Alaseel SE, Fita IZ, Khalid MS, Pottoo FH (2019) Preparation of a novel curcumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation. RSC Adv 9(35):20192–20206
Tran HN, Chao HP (2018) Adsorption and desorption of potentially toxic metals on modified biosorbents through new green grafting process. Environ Sci Pollut Res 25:12808–12820
Haddad MR, El Mamouni, Saffaj N, Lazar S (2018) Removal of a cationic dye–basic Red 12–from aqueous solution by adsorption onto animal bone meal. JAAUBAS 12(1):48–54
Elwakeel KZ, El Bindary AA, Kouta EY (2017) Retention of copper, cadmium and lead from water by Na-Y-Zeolite confined in methyl methacrylate shell. J Environ Chem En 5:3698–3710
Hasani T, Eisazadeh H (2013) Removal of Cd(II) by using polypyrrole and its nanocomposites. Synth Metals 175:15–30
Al-Qahtani KM (2016) Water purification using different waste fruit cortexes for the removal of heavy metals. JTUSCI 10:700–708
Sukriti J, Sharma CAS, Pruthi V, Anand P, Bhatia J, Kaith BS (2017) Sequestration of dyes from artificially prepared textile effluent using RSM-CCD optimized hybrid backbone based adsorbent-kinetic and equilibrium studies. J Environ Manage 190:176–187
Anfar Z, El Haouti R, Lhanafi S, Benafqir M, Azougarh Y, El Alem N (2017) Treated digested residue during anaerobic co-digestion of Agri-food organic waste: methylene blue adsorption, mechanism and CCD-RSM design. J Environ Chem Eng 5(6):5857–5867
Makinde OE, Ajai AI (2015) Adsorption study of lead(II) from aqueous solution by modified and unmodified chitosan. Cont J Appl Sci 10:1–10
Esmael AI, Matta ME, Halim HA, Abdel Azziz FM (2014) Adsorption of heavy metals from industrial wastewater using palm date pits as low cost adsorbent. Int J Eng Adv Technol (IJEAT) 3:71–76
Baroni P, Vieira RS, Meneghetti E, Da Silva MGC, Beppu MM (2008) Evaluation of batch adsorption of chromium ions on natural and crosslinked chitosan membranes. J Hazard Mater 152:1155–1163
Singh TS, Pant KK (2004) Equilibrium, kinetics and thermodynamic studies for adsorption of As(III) on activated alumina. Sep Purif Technol 36:139–147
Thilagan J, Gopalakrishnan S, Kannadasan T (2013) A study on adsorption of Copper(II) ions in aqueoussolution by Chitosan reinforced by Banana stem fibre. Int J Green and Herbal Chem 2:226–240
Yalcin S (2014) The mechanism of heavy metal biosorption on green marine macroalga Enteromorpha linza. CLEAN Soil Air Water 42:251–259
Freundlich HMF (1906) Uber die adsorption in losungen. Int J Res Phys Chem 57:385–470
Voice TC, Weber WJ (1983) Sorption of hydraulic compounds by sediments, soilsand suspended solids—I, theory and background. Water Res 17:1433–1441
Tofan L, Wenkert R, Paduraru CC (2016) Natural and waste materials as green sorbents for Cd(ll) removal from aqueous effluents. Environ Eng Manage J 15:1049–1058
Ngah WSW, Fatinathan S (2008) Adsorption of Cu(II) ions in aqueous solution using chitosan beads, chitosan–GLA beads and chitosan–alginate beads. Chem Eng J 143:62–72
Zhu HY, Fu YQ, Jiang R, Yao J, Xiao L, Zeng GM (2012) Novel magnetic chitosan/poly(vinyl alcohol) hydrogel beads: Preparation, characterization and application for adsorption of dye from aqueous solution. Bioresour Technol 105:24–30
Rahman MS, Sathasivam KV (2015) Heavy metal adsorption onto Kappaphycus sp. from aqueous solutions: the use of error functions for validation of isotherm and kinetics models. Hindawi Publishing Corporation. BioMed Res Int 13:25–35
Pant K, Singh TS (2004) Equilibrium, kinetics, and thermodynamics, study for adsorption of As(III) ions on activated alumina. Sep Purif Technol 36:139–147
Li Y, Du Q, Wang X, Zhang P, Wang D, Wang Z, Xia Y (2010) Removal of Pb from aqueous solution by activated carbon prepared from Enteromorpha prolifera by ZnCl2 activation. J Hazard Mater 183:583–589
Zhang C, Qineng P, Zhang HJ (2004) Self-assembly and characterization of paclitaxel-loaded N-octyl-O-sulfate chitosan micellar system. Colloids Surf B Biointerfaces 39:69–75
Zhu Q, Wang Y, Li M, Liu K, Hu C, Yan K, Sun G (2017) Separation and Activable carboxylic acid functionalized crystalline nanocellulose/PVA-co-PE composite nanofibrous membrane with enhanced adsorption for heavy metal ions. Sep Purif Technol 186:70–71
Zhu X, Cui Y, Chang X, Wang H (2016) Selective solid-phase extraction and analysis of trace-level Cr(III), Fe(III), Pb(II), and Mn(II) ions in wastewater using diethylenetriamine-functionalized carbon nanotubes dispersed in graphene oxide colloids. Talanta 146:358–436
Adegboye MF, Babalola OO (2013) Phylogenetic characterization of culturable antibiotic producing streptomyces from Rhizospheric. Soils Mol Biol 1:34–42
Han J, Zhou Z, Yin R, Yang D, Nie J (2010) Alginate–chitosan/hydroxyapatite polyelectrolyte complex porous scaffolds: preparation and characterization. Int J Biol Macromol 46:199–205
Sudarshan NR, Hoover DG, Knorr D (1992) Antibacterial action of chitosan. Food Biotechnol 6:257–272
Helander IM, Von Wright A, Mattila-Sandholm TM (1997) Potential of lactic acid bacteria and novel antimicrobials against gram-negative bacteria. Trend Food Sci Technol 8:146–150
Helander IM, Lassila ELN, Ahvenainen R, Rhoades J, Roller S (2001) Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int J Food Microbiol 71:235–244
Kong M, Chen X, Xing K, Park H (2010) Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol 144:51–63
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Radha, E., Gomathi, T., Sudha, P.N. et al. Cadmium(II) ion removal from aqueous solution using chitosan oligosaccharide-based blend. Polym. Bull. 78, 1109–1132 (2021). https://doi.org/10.1007/s00289-020-03136-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03136-0