Abstract
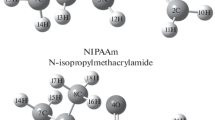
This paper describes the theoretical comparison of the reactivity as comonomers of some nitrogen-containing monomers. Theoretical calculations of monomers commonly used as comonomer in preparation of micro- or nano-gels were carried out by using CAM-B3LYP/6-31+G and B3LYP/6-311G+ (d,p) basis sets of density functional theory. The vibrational frequencies and chemical shifts were calculated by both methods and compared with each other. VEDA 4 program was used for detailed frequency analysis. HOMO and LUMO analysis, electronic properties and NBO analysis are also determined using the same methods and compared. NBO analysis confirms the delocalization of electron density within the monomers. In addition, molecular electrostatic potential maps were generated for each monomer to identify reactive regions. Finally, thermodynamic functions and Mulliken atomic charges were calculated, and the results calculated by both methods matched well.







Similar content being viewed by others
References
Kroschwitz JI (1989) Polymers: biomaterials and medical applications. Wiley, New York
Lee JW, Park JH, Robinson JR (2000) Bioadhesive-based dosage forms: the next generation. J Pharm Sci 89(7):850–866
Haaf F, Sanner A, Straub F (1985) Polymers of N-vinylpyrrolidone: synthesis, characterization and uses. Polym J 17(1):143–152
Duan DC (1993) Pressure-sensitive adhesives. U.S. Patent no. 5,225,473, U.S. Patent and Trademark Office, Washington, DC
Gaenger K, Florig E (2007) Hair treatment composition containing a combination of three different film-forming hair-fixing polymers. U.S. Patent no. 7,279,153, U.S. Patent and Trademark Office, Washington, DC
Suzuki T, Fukuda M, Yoneto K (1995) Pharmaceutical preparation for precutaneous absorption. U.S. Patent no. 5,413,776, U.S. Patent and Trademark Office, Washington, DC
Nho YC, Park KR (2002) Preparation of properties of PVA/PVP hydrogel containing chitosan by radiation. J Appl Polym Sci 85(8):1787–1794
Nud’ga LA, Petrova VA, Klishevich NV, Litvinova LS, Babenko AY, Shelegedin VN (2002) Synthesis and microbiological stability of graft copolymers of N-vinylpyrrolidone and chitosan. Russ J Appl Chem 75(10):1678–1682
Molyneux P (2018) Water-soluble synthetic polymers: volume II: properties and behavior. CRC Press, Boca Raton
Sahiner N, Yasar AO (2013) The generation of desired functional groups on poly(4-vinyl pyridine) particles by post-modification technique for antimicrobial and environmental applications. J Colloid Interface Sci 402:327–333
Rivas BL, Maturana HA, Molina MJ, Gómez-Aantón MR, Piérola IF (1998) Metal ion binding properties of poly(N-vinylimidazole) hydrogels. J Appl Polym Sci 67(6):1109–1118
Suzuki M, Kobayashi S, Kimura M, Hanabusa K, Shirai H (1997) Hydrogen generation using water-insoluble polymer-boundruthenium(II) complexes. Chem Commun 2:227–228
Yoshida S, Ishida H (1983) A study on the orientation of imidazoles on copper as corrosion inhibitor and possible adhesion promoter for electric devices. J Chem Phys 78(11):6960–6969
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27(2):195–226
Wahdan MH, Gomma GK (1997) Effect of copper cation on electrochemical behaviour of steel in presence of imidazole in acid medium. Mater Chem Phys 47(2–3):176–183
Akman F (2016) Experimental and theoretical investigation of molecular structure, vibrational analysis, chemical reactivity, electrostatic potential of benzyl methacrylate monomer and homopolymer. Can J Phys 94(9):853–864
Demir P, Akman F (2017) Molecular structure, spectroscopic characterization, HOMO and LUMO analysis of PU and PCL grafted onto PEMA-co-PHEMA with DFT quantum chemical calculations. J Mol Struct 1134:404–415
Akman F, Çankaya N (2016) A study of experimental and theoretical analysis of N-cyclohexylmethacrylamide monomer based on DFT and HF computations. Pigment Resin Technol 45(5):301–307
Akman F (2017) Molecular structure, kinetics and mechanism of thermal decomposition, molecular electrostatic potential, thermodynamic parameters and HOMO–LUMO analysis of coumarin-containing graft copolymer. Polym Bull 74(8):2975–2993
Frisch MJ, Trucks GW, Schlegel HB, Schlegel GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta Jr and JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J and Fox DJ (2010) Gaussian Inc, Wallingford
Dennington R, Keith T, Millam J (2010) Gauss view, version 5. Semichem Inc., Shawnee Mission
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38(6):3098
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393(1–3):51–57
Tawada Y, Tsuneda T, Yanagisawa S, Yanai T, Hirao K (2004) A long-range-corrected time-dependent density functional theory. J Chem Phys 120(18):8425–8433
Jamroz MH (2004–2010) Vibrational energy distribution analysis VEDA 4, Warsaw
Parr RG, Donnelly RA, Levy M, Palke WE (1978) Electronegativity: the density functional viewpoint. J Chem Phys 68(8):3801–3807
Chattaraj PK, Maiti B, Sarkar U (2003) Philicity: a unified treatment of chemical reactivity and selectivity. J Phys Chem A 107(25):4973–4975
Parr RG, Szentpaly LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121(9):1922–1924
Foster JP, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102(24):7211–7218
Scrocco E, Tomasi J (1973) The electrostatic molecular potential as a tool for the interpretation of molecular properties. New concepts II, Topics Current Chemistry, vol 42. Springer, Berlin, pp 95–170
Luque FJ, López JM, Orozco M (2000) Perspective on “Electrostatic interactions of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects. Theor Chem Acc 103(3–4):343–345
Mehrdad A, Taleb-Abbasi M, Rezaei M (2019) Miscibility behavior of hydroxyethyl cellulose/poly(vinyl pyrrolidone) blends in the presence of some imidazolium based ionic liquids. J Mol Liq 296:111844
D’Amelia RP, Gentile S, Nirode WF, Huang L (2016) Quantitative analysis of copolymers and blends of polyvinyl acetate (PVAc) using Fourier transform infrared spectroscopy (FTIR) and elemental analysis (EA). World J Chem Educ 4(2):25–31
Kaur I, Kumari V, Sharma B, Gupta N (2013) Characterization and applications of PVF film grafted with binary mixture of methacrylic acid and 4-vinyl pyridine by gamma radiations: effect of swift heavy ions. Appl Radiat Isot 79:118–130
Wang R, Xiang T, Yue W, Li H, Liang S, Sun S, Zhao C (2012) Preparation and characterization of pH-sensitive polyethersulfone hollow fiber membranes modified by poly(methyl methylacrylate-co-4-vinyl pyridine) copolymer. J Membr Sci 423:275–283
El-Hoshoudy AN, Desouky SEM, Betiha MA, Alsabagh AM (2016) Use of 1-vinyl imidazole based surfmers for preparation of polyacrylamide–SiO2 nanocomposite through aza-Michael addition copolymerization reaction for rock wettability alteration. Fuel 170:161–175
Kuba AG, Smolin YY, Soroush M, Lau KK (2016) Synthesis and integration of poly(1-vinylimidazole) polymer electrolyte in dye sensitized solar cells by initiated chemical vapor deposition. Chem Eng Sci 154:136–142
Tripathi A, Srivastava AK (2008) Studies on the radical polymerization of N-vinyl 2-pyrrolidone initiated by diphenyl ditelluride. J Polym Res 15(3):187–193
Mulliken RS (1955) Electronic population analysis on LCAO–MO molecular wave functions. I. J Chem Phys 23(10):1833–1840
Acknowledgements
The author would like to thank Bingöl University for the server and Bitlis Eren University for Gaussian software.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akman, F. A comparative study based on molecular structure, spectroscopic, electronic, thermodynamic and NBO analysis of some nitrogen-containing monomers. Polym. Bull. 78, 663–693 (2021). https://doi.org/10.1007/s00289-020-03128-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03128-0