Abstract
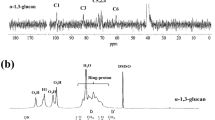
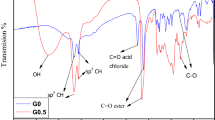
A novel 2.5th-generation glycodendritic polyamine dextran with 96 arms (DPADx) was synthesized by the divergent method. 2.5th-generation dendrimer was obtained from the successive triplicate Michael addition with two amidation reactions using 0.5th-generation tris(2-aminoethyl)amine (TAEA-G0.5) as the initiator core. 2.5th-generation polyamine hydrazide (PAH) was obtained from hydrazine monohydrate. Finally, dextran was conjugated with PAH and so DPADx was obtained. FTIR, 1H- and 13C-NMR, elemental analysis, GPC, XRD, SEM, TGA, DSC and dynamic viscosity were used for structural analysis of products. Loading efficiency, capacity and yield (%) of eugenol-encapsulated glycodendrimer were calculated as 70%, 84% and 35%, respectively; by using UV–Vis analysis results, antimicrobial activity against Gram-positive (Staphylococcus aureus, Listeria monocytogenes and Enterococcus cesseliflour) and Gram-negative (Escherichia coli and Salmonella Typhimurium) bacteria was proven. The current study demonstrated the usability of the novel dendritic glycopolymer as an innovative and environmentally friendly way to encapsulate and shuttle of EOs without altering sensory characteristics and the functionality of these molecules. Therefore, its commercial application is possible in a variety of fields, such as pharmaceutic and cosmetic industries.






Similar content being viewed by others
References
Franz C, Novak J (2010) Sources of essential oils. In: Baser KHC, Buvhbauer G (eds) Handbook of essential oils: science, technology and applications. CRC Press, Boca Raton, pp 39–82
Acik G (2019) Soybean oil modified bio-based poly(vinyl alcohol)s via ring-opening polymerization. J Polym Environ 27:2618–2623. https://doi.org/10.1007/s10924-019-01547-3
Silvestre W, Livinalli N, Baldasso C, Tessaro I (2019) Pervaporation in the separation of essential oil components: a review. Trends Food Sci Technol 93:42–52. https://doi.org/10.1016/j.tifs.2019.09.003
Dima C, Dima S (2015) Essential oils in foods: extraction, stabilization, and toxicity. Curr Opin Food Sci 5:29–35
Laosinwattana C, Wichittrakarn P, Teerarak M (2018) Chemical composition and herbicidal action of essential oil from Tagetes erecta L. leaves. Ind Crops Prod 126:129–134. https://doi.org/10.1016/j.indcrop.2018.10.013
Mourtzinos I, Kalogeropoulos N, Papadakis S et al (2007) Encapsulation of nutraceutical monoterpenes in β-cyclodextrin and modified starch. J Food Sci. https://doi.org/10.1111/j.1750-3841.2007.00609.x
Turek C, Stintzing FC (2013) Stability of essential oils: a review. Compr Rev Food Sci Food Saf 12:40–53. https://doi.org/10.1111/1541-4337.12006
Biduski B, Kringel DH, Colussi R et al (2019) Electrosprayed octenyl succinic anhydride starch capsules for rosemary essential oil encapsulation. Int J Biol Macromol 132:300–307. https://doi.org/10.1016/j.ijbiomac.2019.03.203
Ferrer MCC, Dastgheyb S, Hickok NJ et al (2014) Designing nanogel carriers for antibacterial applications. Acta Biomater 10:2105–2111. https://doi.org/10.1016/j.actbio.2014.01.009
Newkome GR, Shreiner CD (2008) Poly(amidoamine), polypropylenimine, and related dendrimers and dendrons possessing different 1 → 2 branching motifs: an overview of the divergent procedures. Polymer 49:1–173. https://doi.org/10.1016/j.polymer.2007.10.021
Huang D, Wu D (2018) Biodegradable dendrimers for drug delivery. Mater Sci Eng C 90:713–727. https://doi.org/10.1016/j.msec.2018.03.002
Sherje AP, Jadhav M, Dravyakar BR, Kadam D (2018) Dendrimers: a versatile nanocarrier for drug delivery and targeting. Int J Pharm 548:707–720. https://doi.org/10.1016/j.ijpharm.2018.07.030
Tomalia DA, Naylor AM, Goddard WA (1990) Starburst dendrimers: molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew Chem Int Ed Engl 29:138–175. https://doi.org/10.1002/anie.199001381
Lyu Z, Ding L, Huang AY-T, Kao C-L, Peng L (2019) Poly(amidoamine) dendrimers: covalent and supramolecular. Synth Mater Today Chem 13:34–48. https://doi.org/10.1016/j.mtchem.2019.04.004
Coimbra M, Isacchi B, Bloois LV et al (2011) Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int J Pharm 416:433–442. https://doi.org/10.1016/j.ijpharm.2011.01.056a<
Mukherji SP (1995) Ocimum-a cheap source of eugenol. Science reporter, 31, 599. Eugenol-rich Ocimum variety released. In: CSIR NEWS Published by PID CSIR, New Delhi, 45, 256
Talón E, Vargas M, Chiralt A, González-Martínez C (2019) Antioxidant starch-based films with encapsulated eugenol. Application to sunflower oil preservation. LWT 113:108290. https://doi.org/10.1016/j.lwt.2019.108290
Gomes D, Pinto JC, Borges C (2003) Determination of hydrazide content in poly(oxadiazole-hydrazide) copolymers by NMR and thermal analysis. Polymer 44:6223–6233. https://doi.org/10.1016/s0032-3861(03)00638-4
Zhu L, Shi Y, Tu C et al (2010) Construction and application of a pH-sensitive nanoreactor via a double-hydrophilic multiarm hyperbranched polymer. Langmuir 26:8875–8881. https://doi.org/10.1021/la9046275
Liu C, Lin Q, Gao Y et al (2007) Characterization and antitumor activity of a polysaccharide from Strongylocentrotus nudus eggs. Carbohydr Polym 67:313–318. https://doi.org/10.1016/j.carbpol.2006.05.024
Hammer KA, Carson CF, Riley TV (1999) Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol 86:985–990. https://doi.org/10.1046/j.1365-2672.1999.00780.x
Klaykruayat B, Siralertmukul K, Srikulkit K (2010) Chemical modification of chitosan with cationic hyperbranched dendritic polyamidoamine and its antimicrobial activity on cotton fabric. Carbohydr Polym 80:197–207. https://doi.org/10.1016/j.carbpol.2009.11.013
Liu X, Liu J, Luo Y (2012) Facile glycosylation of dendrimers for eliciting specific cell–material interactions. Polym Chem 3:310–313. https://doi.org/10.1039/c1py00404b
Purama RK, Goswami P, Khan AT, Goyal A (2009) Structural analysis and properties of dextran produced by Leuconostoc mesenteroides NRRL B-640. Carbohydr Polym 76:30–35. https://doi.org/10.1016/j.carbpol.2008.09.018
Shingel KI (2002) Determination of structural peculiarities of dexran, pullulan and γ-irradiated pullulan by Fourier-transform IR spectroscopy. Carbohydr Res 337:1445–1451. https://doi.org/10.1016/s0008-6215(02)00209-4
Charles S, Vasanthan N, Kwon D et al (2012) Surface modification of poly(amidoamine) (PAMAM) dendrimer as antimicrobial agents. Tetrahedron Lett 53:6670–6675. https://doi.org/10.1016/j.tetlet.2012.09.098
Gajendiran M, Gopi V, Elangovan V et al (2013) Isoniazid loaded core shell nanoparticles derived from PLGA–PEG–PLGA tri-block copolymers: in vitro and in vivo drug release. Colloids Surf B 104:107–115. https://doi.org/10.1016/j.colsurfb.2012.12.008
Zhao D, Ong S-M, Yue Z et al (2008) Dendrimer hydrazides as multivalent transient inter-cellular linkers. Biomaterials 29:3693–3702. https://doi.org/10.1016/j.biomaterials.2008.05.019
Gomes C, Moreira RG, Castell-Perez E (2011) Poly(DL-lactide-co-glycolide) (PLGA) nanoparticles with entrapped trans-cinnamaldehyde and eugenol for antimicrobial delivery applications. J Food Sci. https://doi.org/10.1111/j.1750-3841.2010.01985.x
Siddiqui NN, Aman A, Silipo A et al (2014) Structural analysis and characterization of dextran produced by wild and mutant strains of Leuconostoc mesenteroides. Carbohydr Polym 99:331–338. https://doi.org/10.1016/j.carbpol.2013.08.004
Zhang J, Shan D, Mu S (2007) A promising copolymer of aniline and m-aminophenol: chemical preparation, novel electric properties and characterization. Polymer 48:1269–1275. https://doi.org/10.1016/j.polymer.2006.12.021
Schmidt-Rohr K, Kulik AS, Beckham HW et al (1994) Molecular nature of the.beta. relaxation in poly(methyl methacrylate) investigated by multidimensional NMR. Macromolecules 27:4733–4745. https://doi.org/10.1021/ma00095a014
Wang K-R, An H-W, Rong R-X et al (2014) Synthesis of biocompatible glycodendrimer based on fluorescent perylene bisimides and its bioimaging. Macromol Rapid Commun 35:727–734. https://doi.org/10.1002/marc.201300916
Frechet J (1994) Functional polymers and dendrimers: reactivity, molecular architecture, and interfacial energy. Science 263:1710–1715. https://doi.org/10.1126/science.8134834
Oliveira EFD, Paula HC, Paula RCD (2014) Alginate/cashew gum nanoparticles for essential oil encapsulation. Colloids Surf B 113:146–151. https://doi.org/10.1016/j.colsurfb.2013.08.038
Chung H-J, Lim S-T (2003) Physical aging of glassy normal and waxy rice starches: effect of aging temperature on glass transition and enthalpy relaxation. Carbohydr Polym 53:205–211. https://doi.org/10.1016/s0144-8617(03)00077-8
Icoz DZ, Moraru CI, Kokini JL (2005) Polymer–polymer interactions in dextran systems using thermal analysis. Carbohydr Polym 62:120–129. https://doi.org/10.1016/j.carbpol.2005.07.012
Karpagam S, Guhanathan S (2014) Phosphorus based indole and imidazole functionalized hyperbranched polyester as antimicrobial surface coating materials. Prog Org Coat 77:1901–1910. https://doi.org/10.1016/j.porgcoat.2014.06.022
Azmeera V, Adhikary P, Krishnamoorthi S (2012) Synthesis and characterization of graft copolymer of dextran and 2-acrylamido-2-methylpropane sulphonic acid. Int J Carbohydr Chem 2012:1–7. https://doi.org/10.1155/2012/209085
Chen H, Zhang Y, Zhong Q (2015) Physical and antimicrobial properties of spray-dried zein–casein nanocapsules with co-encapsulated eugenol and thymol. J Food Eng 144:93. https://doi.org/10.1016/j.jfoodeng.2014.07.021
Woranuch S, Yoksan R (2013) Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydr Polym 96(2):578. https://doi.org/10.1016/j.carbpol.2012.08.117
Hill LE, Gomes C, Taylor TM (2013) Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT Food Sci Technol 51:86–93. https://doi.org/10.1016/j.lwt.2012.11.011
Kürekci C, Yipel M, Önen SP (2016) Investigation of the effectiveness of some plant compounds and essential oils of corymbia citriodora against foodborne pathogens. Turk J Agric Food Sci Technol 4:968. https://doi.org/10.24925/turjaf.v4i11.968-972.853
Acknowledgements
The authors thank Hatay Mustafa Kemal University [Scientific Research Project Unit (BAP), (9980)] for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bucak, C.D., Kürekci, C. & Dinç, C.Ö. Carrying system formula for eugenol encapsulation: glycodendritic polyamine dextran-G2.5, synthesis and in vitro antibacterial activity. Polym. Bull. 78, 601–620 (2021). https://doi.org/10.1007/s00289-020-03125-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03125-3