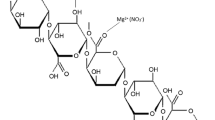
Abstract
Currently, biopolymer electrolytes are attracting a great deal of interest as substitute for synthetic polymer electrolytes in electrochemical devices, as they are carbon neutral, sustainable, reduce dependency on non-renewable fossil fuels and easily biodegradable. Some of the biopolymers under research are chitosan, pectin, agar–agar, cellulose acetate and carrageenan. The current work deals with the study of ion conducting polymer electrolyte, pectin with magnesium chloride salt for magnesium battery application. Biopolymer electrolytes of different compositions of pectin with different concentrations of magnesium chloride salt are prepared by solution casting technique and subjected to various studies like by X-ray diffraction (XRD), Fourier transform infrared (FTIR), differential scanning calorimetry (DSC), AC impedance spectroscopy and linear sweep voltammetry (LSV). XRD analysis has been used to identify the amorphous/crystalline nature of the sample. The complex formation between the polymer pectin and the magnesium chloride salt has been analyzed by FTIR spectroscopy. DSC analysis is a thermo-analytical technique which is used to observe the glass transition temperature (Tg) of the samples. AC impedance technique has been used to find the ionic conductivities of the sample. The electrochemical stability of the polymer electrolyte has been analyzed by linear sweep voltammetry. Among the prepared polymer electrolytes, 30 M wt% pectin: 70 M wt% MgCl2 offers the highest ionic conductivity of 1.14 × 10−3 S cm−1. The electrochemical stability of the highest conducting sample is 2.05 V. The primary magnesium battery has been constructed using the highest conducting sample, 30 M wt% pectin: 70 M wt% MgCl2, and the battery performance has been studied.












Similar content being viewed by others
References
Malathi M, Tamilarasan K, Ganesan V (2014) Role of ceramic reinforcement in composite polymer electrolyte. Polym Compos 36(1):42–46. https://doi.org/10.1002/pc.22910
Wang SH, Kuo PL, Hsieh CT, Teng H (2014) Design of poly(acrylonitrile)-based gel electrolytes for high-performance lithium ion batteries. ACS Appl Mater Interfaces 6(21):19360–19370. https://doi.org/10.1021/am505448a
Wang J, Song S, Muchakayala R, Hu X, Liu R (2017) Structural, electrical, and electrochemical properties of PVA-based biodegradable gel polymer electrolyte membranes for Mg-ion battery applications. Ionics 23(7):1759–1769. https://doi.org/10.1007/s11581-017-1988-y
Basha SKS, Sundari GS, Kumar KV, Rao MC (2017) Preparation and dielectric properties of PVP-based polymer electrolyte films for solid-state battery application. Polym Bull 75(3):925–945. https://doi.org/10.1007/s00289-017-2072-5
Wang F, Li L, Yang X, You J, Xu Y, Wang H, Ma Y, Gao G (2018) Influence of additives in a PVDF-based solid polymer electrolyte on conductivity and Li-ion battery performance. Sustain Energy Fuels 2(2):492–498. https://doi.org/10.1039/c7se00441a
Yulianti E, Karo AK, Susita L, Sudaryanto (2012) Synthesis of electrolyte polymer based on natural polymer chitosan by ion implantation technique. Proc Chem 4:202–207. https://doi.org/10.1016/j.proche.2012.06.028
Zulkefli FN, Navaratnam S, Ahmad AH (2015) Proton conducting biopolymer electrolytes based on starch incorporated with ammonium thiocyanate. Adv Mater Res 1112:275–278. https://doi.org/10.4028/www.scientific.net/AMR.1112.275
Khiar ASA, Arof AK (2009) Conductivity studies of starch-based polymer electrolytes. Ionics 16(2):123–129. https://doi.org/10.1007/s11581-009-0356-y
Moniha V, Alagar M, Selvasekarapandian S, Sundaresan B, Hemalatha R, Boopathi G (2018) Synthesis and characterization of bio-polymer electrolyte based on iota-carrageenan with ammonium thiocyanate and its applications. J Solid State Electrochem. https://doi.org/10.1007/s10008-018-4028-6
Moniha V, Alagar M, Selvasekarapandian S, Sundaresan B, Boopathi G (2018) Conductive bio-polymer electrolyte iota-carrageenan with ammonium nitrate for application in electrochemical devices. J Non-Cryst Solids 481:424–434. https://doi.org/10.1016/j.jnoncrysol.2017.11.027
Chitra R, Sathya P, Selvasekarapandian S, Monisha S, Moniha V, Meyvel S (2018) Synthesis and characterization of iota-carrageenan solid biopolymer electrolytes for electrochemical applications. Ionics 25(5):2147–2157. https://doi.org/10.1007/s11581-018-2687-z
Christopher Selvin P, Perumal P, Selvasekarapandian S, Monisha S, Boopathi G, Leena Chandra MV (2018) Study of proton-conducting polymer electrolyte based on K-carrageenan and NH4SCN for electrochemical devices. Ionics 24:3535–3542. https://doi.org/10.1007/s11581-018-2521-7
Isa MIN, Samsudin AS (2016) Potential study of biopolymer-based carboxymethylcellulose electrolytes system for solid-state battery application. Int J Polym Mater Polym Biomater 65(11):561–567. https://doi.org/10.1080/00914037.2016.1149844
Rani M, Rudhziah S, Ahmad A, Mohamed N (2014) Biopolymer electrolyte based on derivatives of cellulose from kenaf bast fiber. Polymers 6(9):2371–2385. https://doi.org/10.3390/polym6092371
Raphael E, Avellaneda CO, Manzolli B, Pawlicka A (2010) Agar-based films for application as polymer electrolytes. Electrochim Acta 55(4):1455–1459. https://doi.org/10.1016/j.electacta.2009.06.010
Kumar LS, Selvin PC, Selvasekarapandian S, Manjuladevi R, Monisha S, Perumal P (2018) Tamarind seed polysaccharide biopolymer membrane for lithium-ion conducting battery. Ionics 24(12):3793–3803. https://doi.org/10.1007/s11581-018-2541-3
Thakur BR, Singh RK, Handa AK, Rao MA (1997) Chemistry and uses of pectin—a review. Crit Rev Food Sci Nutr 37(1):47–73. https://doi.org/10.1080/10408399709527767
Perumal P, Selvasekarapandian S, Abhilash KP, Sivaraj P, Hemalatha R, Selvin PC (2019) Impact of lithium chlorate salts on structural and electrical properties of natural polymer electrolytes for all solid state lithium polymer batteries. Vacuum 159:277–281. https://doi.org/10.1016/j.vacuum.2018.10.043
Muthukrishnan M, Shanthi C, Selvasekarapandian S, Manjuladevi R, Perumal P, Christopher Selvin P (2019) Synthesis and characterization of pectin-based biopolymer electrolyte for electrochemical applications. Ionics 25(1):203–214. https://doi.org/10.1007/s11581-018-2568-5
Vijaya N, Selvasekarapandian S, Somalatha M, Sujithra KS, Monisha S (2016) Proton-conducting biopolymet electrolytes based on pectin doped with NH4X (X = Cl, Br). Ionics 23(10):2799–2808. https://doi.org/10.1007/s11581-016-1852-5
Kavitha S, Vijaya N, Pandeeswari R, Premalatha M (2016) Vibrational, electrical and optical studies on pectin-based polymer electrolyte. Int Res J Eng Technol 3(7):1385–1390
Mendes JP, Esperança JMSS, Medeiros MJ, Pawlicka A, Silva MM (2017) Structural, morphological, ionic conductivity, and thermal properties of pectin-based polymer electrolytes. Mol Cryst Liq Cryst 643(1):266–273. https://doi.org/10.1080/15421406.2016.1263111
Mahalakshmi M, Selvanayagam S, Selvasekarapandian S, Moniha V, Manjuladevi R, Sangeetha P (2019) Characterization of biopolymer electrolytes based on cellulose acetates with magnesium perchlorate (Mg(ClO4)2) for energy storage devices. J Sci Adv Mater Devices 4(2):276–284. https://doi.org/10.1016/j.jsamd.2019.04.006
Perumal P, Abhilash KP, Sivaraj P, Selvin PC (2019) Study on Mg-ion conducting solid biopolymer electrolytes based on tamarind seed polysaccharide for magnesium ion batteries. Mater Res Bull. https://doi.org/10.1016/j.materresbull.2019.05.015
Priya SS, Karthika M, Selvasekarapandian S, Manjuladevi R, Monisha S (2018) Study of biopolymer I-carrageenan with magnesium perchlorate. Ionics 24(12):3861–3875. https://doi.org/10.1007/s11581-018-2535-1
Priya SS, Karthika M, Selvasekarapandian S, Manjuladevi R (2018) Preparation and characterization of polymer electrolyte based on biopolymer I-carrageenan with magnesium nitrate. Solid State Ion 327(1):136–149. https://doi.org/10.1016/j.ssi.2018.10.031
Manjuladevi R, Selvasekarapandian S, Thamilselvan M, Mangalam R, Monisha S, Selvin PC (2018) A study on blend polymer electrolyte based on poly(vinyl alcohol)-poly(acrylonitrile) with magnesium nitrate for magnesium battery. Ionics 24(11):3493–3506. https://doi.org/10.1007/s11581-018-2500-z
Manjuladevi R, Thamilselvan M, Selvasekarapandian S, Christopher Selvin P, Mangalam R, Monisha S (2017) Preparation and characterization of blend polymer electrolyte film based on poly(vinyl alcohol)-poly(acrylonitrile)/MgCl2 for energy storage devices. Ionics 24(4):1083–1095. https://doi.org/10.1007/s11581-017-2273-9
Leones R, Botelho MBS, Sentanin F, Cesarino I, Pawlicka A, Camargo ASS, Silva MM (2014) Pectin-based polymer electrolytes with Ir(III) complexes. Mol Cryst Liq Cryst 604(1):117–125. https://doi.org/10.1080/15421406.2014.968049
Andrade JR, Raphael E, Pawlicka A (2009) Plasticized pectin based gel electrolytes. Electrochim Acta 54(26):6479–6483. https://doi.org/10.1016/j.electacta.2009.05.098
Kiruthika S, Malathi M, Selvasekarapandian S, Tamilarasan K, Moniha V, Manjuladevi R (2019) Eco-friendly biopolymer electrolyte, pectin with magnesium nitrate salt, for application in electrochemical devices. J Solid State Electrochem 23(7):2181–2193. https://doi.org/10.1007/s10008-019-04313-6
Hodge RM, Edward GH, Simon GP (1996) Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 37(8):1371–1376. https://doi.org/10.1016/0032-3861(96)81134-7
Maciel VBV, Yoshida CMP, Franco TT (2015) Chitosan/pectin polyelectrolyte complex as a pH indicator. Carbohydr Polym 132(5):537–545. https://doi.org/10.1016/j.carbpol.2015.06.047
Perumal P, Christopher Selvin P, Selvasekarapandian S (2018) Characterization of biopolymer pectin with lithium chloride and its applications to electrochemical devices. Ionics 24(10):3259–3270. https://doi.org/10.1007/s11581-018-2507-5
Nirmala Devi G, Chitra S, Selvasekarapandian S, Premalatha M, Monisha S, Saranya J (2017) Synthesis and characterization of dextrin-based polymer electrolytes for potential applications in energy storage devices. Ionics 23(12):3377–3388. https://doi.org/10.1007/s11581-017-2135-5
Premalatha M, Vijaya N, Selvasekarapandian S, Selvalakshmi S (2016) Characterization of blend polymer PVA–PVP complexed with ammonium thiocyanate. Ionics 22(8):1299–1310. https://doi.org/10.1007/s11581-016-1672-7
Boukamp BA (1986) A non-linear lease square fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion 20(1):31–44. https://doi.org/10.1016/0167-2738%2886%2990031-7
Ravi M, Song S, Wang J, Wang T, Nadimicherla R (2016) Ionic liquid incorporated biodegradable gel polymer electrolyte for lithium ion battery applications. J Mater Sci: Mater Electron 27(2):1370–1377. https://doi.org/10.1007/s10854-015-3899-x
Wagner JB, Wagner C (1957) Electrical conductivity measurements on cuprous halides. J Chem Phys 26(6):1597–1601. https://doi.org/10.1063/1.1743590
Evans J, Vincent CA, Bruce PG (1987) Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28(13):2324–2328. https://doi.org/10.1016/0032-3861(87)90394-6
Kumar GG, Munichandraiah N (2002) Poly (methylmethacrylate)—magnesium triflate gel polymer electrolyte for solid state magnesium battery application. Electrochim Acta 47(7):1013–1022. https://doi.org/10.1016/S0013-4686(01)00832-5
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kiruthika, S., Malathi, M., Selvasekarapandian, S. et al. Conducting biopolymer electrolyte based on pectin with magnesium chloride salt for magnesium battery application. Polym. Bull. 77, 6299–6317 (2020). https://doi.org/10.1007/s00289-019-03071-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-03071-9