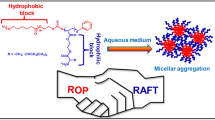
Abstract
Amphiphilic block copolymers where hydrophobicity and hydrophilicity coincide are essential building blocks for many supramolecular systems. By this time, polyethylene glycol (PEG) has been a conventional choice to constitute hydrophilicity; however, it suffers from certain drawbacks, severely limiting its use in these compounds. To date, one potential modality to overcome this complication is to utilize poly(2-ethyl-2-oxazoline) (PEtOx) instead, given that this also-hydrophilic polymer is very comparable to PEG, in many ways. In this regard, amphiphilic block copolymers harboring PEtOx and synthetic approaches to access these polymeric materials have been documented in the literature. Within this scope, we crafted a modular approach for the synthesis of poly(2-ethyl-2-oxazoline)-b-poly(ε-caprolactone) to govern its molecular structure. Herein, we extend this work and report a novel poly(2-ethyl-2-oxazoline)-b-poly(ε-caprolactone) derivative with electrophilic moiety on terminal position. We believe that this novel design could lead up to expeditious synthesis of block copolymer-biomolecule conjugates, which are of paramount significance for many applications.
Graphic abstract








Similar content being viewed by others
References
Nishiyama N, Bae Y, Miyata K, Fukushima S, Kataoka K (2005) Smart polymeric micelles for gene and drug delivery. Drug Discov Today Technol 2(1):21–26. https://doi.org/10.1016/j.ddtec.2005.05.007
Jain JP, Ayen WY, Kumar N (2011) Self assembling polymers as polymersomes for drug delivery. Curr Pharm Des 17:65–79. https://doi.org/10.2174/138161211795049822
Klaikherd A, Nagamani C, Thayumanavan S (2009) Multi-stimuli sensitive amphiphilic block copolymer assemblies. J Am Chem Soc 131(13):4830–4838. https://doi.org/10.1021/ja809475a
Förster S, Plantenberg T (2002) From self-organizing polymers to nanohybrid and biomaterials. Angew Chem Int Ed 41:689–714. https://doi.org/10.1002/1521-3773(20020301)41:5%3c688:AID-ANIE688%3e3.0.CO;2-3
Jeong B, Bae YH, Kim SW (1999) Thermoreversible gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions. Macromolecules 32:7064–7069. https://doi.org/10.1021/ma9908999
Roberts MJ, Bentley MD, Harris JM (2002) Chemistry for peptide and protein PEGylation. Adv Drug Deliver Rev 54:459–476. https://doi.org/10.1016/S0169-409X(02)00022-4
Harris JM, Chess RB (2003) Effect of PEGylation on pharmaceuticals. Nat Rev Drug Discov 2:214–221. https://doi.org/10.1038/nrd1033
Caliceti P, Veronese FM (2003) Pharmacokinetic and biodistribution properties of poly(ethylene glycol)-protein conjugates. Adv Drug Deliv Rev 55:1261–1277. https://doi.org/10.1016/S0169-409X(03)00108-X
Veronese FM, Pasut G (2005) PEGylation, successful approach to drug delivery. Drug Discov Today 10:1451–1458. https://doi.org/10.1016/S1359-6446(05)03575-0
Milla P, Dosio F, Cattel L (2012) PEGylation of proteins and liposomes: a powerful and flexible strategy to improve the drug delivery. Curr Drug Metab 13:105–119. https://doi.org/10.2174/138920012798356934
Duan J, Liu C, Liang X, Li X, Chen Y, Chen Z, Wang X, Kong D, Li Y, Yang J (2018) Protein delivery nanosystem of six-arm copolymer poly(ε-caprolactone)–poly(ethylene glycol) for long-term sustained release. Int J Nanomed 13:2743–2754. https://doi.org/10.2147/IJN.S161006
Xiao RZ, Zeng ZW, Zhou GL, Wang JJ, Li FZ, Wang AM (2010) Recent advances in PEG–PLA block copolymer nanoparticles. Int J Nanomed 5:1057–1065. https://doi.org/10.2147/IJN.S14912
Wang J, Li S, Han Y, Guan J, Chung S, Wang C, Li D (2018) Poly(ethylene glycol)–polylactide micelles for cancer therapy. Front Pharmacol 9:202. https://doi.org/10.3389/fphar.2018.00202
Kutikov AB, Song J (2015) Biodegradable PEG-based amphiphilic block copolymers for tissue engineering applications. ACS Biomater Sci Eng 1(7):463–480. https://doi.org/10.1021/acsbiomaterials.5b00122
Liu G-Y, Chen C-J, Ji J (2012) Biocompatible and biodegradable polymersomes as delivery vehicles in biomedical applications. Soft Matter 8:8811–8821. https://doi.org/10.1039/C2SM25721A
Veronese FM, Mero A, Pasut G (2009) Protein PEGylation, basic science and biological applications. PEGylated protein drugs: basic science and clinical applications. In: Veronese FM (ed) Milestones in drug therapy. Basel, Birkhäuser, pp 11–31. https://doi.org/10.1007/978-3-7643-8679-5_2
Ulbricht J, Jordan R, Luxenhofer R (2014) On the biodegradability of polyethylene glycol, polypeptoids and poly(2-oxazoline)s. Biomaterials 35:4848–4861. https://doi.org/10.1016/j.biomaterials.2014.02.029
Viegas TX, Bentley MD, Harris JM, Fang Z, Yoon K, Dizman B, Weimer R, Mero A, Pasut G, Veronese FM (2011) Polyoxazoline: chemistry, properties, and applications in drug delivery. Bioconj Chem 22:976–986. https://doi.org/10.1021/bc200049d
Tao L, Liu J, Davis TP (2009) Branched polymer-protein conjugates made from mid-chain-functional P(HPMA). Biomacromolecules 12:2847–2851. https://doi.org/10.1021/bm900678r
Jain S, Hreczuk-Hirst DH, McCormack B, Mital M, Epenetos A, Laing P, Gregoriadis G (2003) Polysialylated insulin: synthesis, characterization and biological activity in vivo. Biochim Biophys Acta 1622:42–49. https://doi.org/10.1016/S0304-4165(03)00116-8
Veronese FM, Sartore L, Caliceti P, Schiavon O, Ranucci E, Ferruti P (1990) Low molecular weight end-functionalized poly(N-vinylpyrrolidinone) for the modification of polypeptide amino groups. J Bioact Compat Polym 5:167–178. https://doi.org/10.1177/088391159400900404
Hoogenboom R (2007) Poly(2-oxazoline)s: alive and kicking. Macromol Chem Phys 208(1):18–25. https://doi.org/10.1002/macp.200600558
Zalipsky S, Hansen CB, Oaks JM, Allen TM (1996) Evaluation of blood clearance rates and biodistribution of poly(2-oxazoline)-grafted liposomes. J Pharm Sci 85:133–137. https://doi.org/10.1021/js9504043
Lee SC, Chang YK, Yoon JS, Kim CH, Kwon IC, Kim YH, Jeong SY (1999) Synthesis and micellar characterization of amphiphilic diblock copolymers based on poly(2-ethyl-2-oxazoline) and aliphatic polyesters. Macromolecules 32:1847–1852. https://doi.org/10.1021/ma981664k
Wiesbrock F, Hoogenboom R, Leenen MAM, Meier MAR, Schubert US (2005) Investigation of the living cationic ring-opening polymerization of 2-methyl-, 2-ethyl-, 2-nonyl-, and 2-phenyl-2-oxazoline in a single-mode microwave reactor. Macromolecules 38:5025–5034. https://doi.org/10.1021/ma0474170
Hoogenboom R, Wiesbrock F, Huang H, Leenen MAM, Thijs HML, Van Nispen SFGM, Van der Loop M, Fustin CA, Jonas AM, Gohy JF, Schubert US (2006) Microwave-assisted cationic ring-opening polymerisation of 2-oxazolines: a powerful method for the synthesis of amphiphilic triblock copolymers. Macromolecules 39:4719–4725. https://doi.org/10.1021/ma060952a
Adams N, Schubert US (2007) Poly(2-oxazolines) in biological and biomedical application contexts. Adv Drug Deliv Rev 59:1504–1520. https://doi.org/10.1016/j.addr.2007.08.018
Mero A, Pasut G, Dalla VL, Fijten MW, Schubert US, Hoogenboom R, Veronese FM (2008) Synthesis and characterization of poly(2-ethyl 2-oxazoline)-conjugates with proteins and drugs: suitable alternatives to peg-conjugates. J Control Release 2:87–95. https://doi.org/10.1016/j.jconrel.2007.10.010
Hoogenboom R (2009) Poly(2-oxazoline)s: a polymer class with numerous potential applications. Angew Chem Int Ed 48(43):7978–7994. https://doi.org/10.1002/anie.200901607
Sedlacek O, Monnery BD, Filippov SK, Hoogenboom R, Hruby M (2012) Poly(2-Oxazoline)s: are they more advantageous for biomedical applications than other polymers? Macromol Rapid Commun 33(19):1648–1662. https://doi.org/10.1002/marc.201200453
Kobayashi S (2012) Polymerization of oxazolines. In: Matyjaszewski K, Möller M (eds) Polymer science: a comprehensive reference, vol 4. Elsevier, Amsterdam, pp 397–426. https://doi.org/10.1016/B978-0-444-53349-4.00110-2
Hoogenboom R (2009) Polyethers and polyoxazolines. In: Dubois P, Coulembier O, Raquez J-M (eds) Handbook of ring-opening polymerization. Wiley, Weinheim, pp 141–164. https://doi.org/10.1002/9783527628407.ch6
Luxenhofer R, Han Y, Schulz A, Tong J, He Z, Kabanov AV, Jordan R (2012) Poly(2-oxazoline)s as polymer therapeutics. Macromol Rapid Commun 33:1613–1631. https://doi.org/10.1002/marc.201200354
Isaacman MJ, Theogarajan L (2013) Poly(oxazoline) block copolymers for biomedical applications. Tailored Polym Archit Pharm Biomed Appl 1135:53–68. https://doi.org/10.1021/bk-2013-1135.ch005
Gulyuz S, Ozkose UU, Kocak P, Telci D, Yilmaz O, Tasdelen MA (2018) In-vitro cytotoxic activities of poly(2-ethyl-2-oxazoline)-based amphiphilic block copolymers prepared by CuAAC click chemistry. Express Polym Lett 12(2):146–158. https://doi.org/10.3144/expresspolymlett.2018.13
Zhang Y, He H, Gao C (2008) Clickable macroinitiator strategy to build amphiphilic polymer brushes on carbon nanotubes. Macromolecules 41:9581–9594. https://doi.org/10.1021/ma801696z
Cai T, Li M, Neoh KG, Kang ET (2013) Surface-functionalizable membranes of polycaprolactone-click-hyperbranched polyglycerol copolymers from combined atom transfer radical polymerization, ring-opening polymerization and click chemistry. J Mater Chem B 1:1304–1315. https://doi.org/10.1039/C2TB00273F
https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Datasheet/ b2059dat.pdf
Britto PJ, Knipling L, Wolff J (2002) the local electrostatic environment determines cysteine reactivity of tubulin. J Biol Chem 277:29018–29027. https://doi.org/10.1074/jbc.M204263200
Gurd FRN (1967) Carboxymethylation. Methods Enzymol 11:532–541. https://doi.org/10.1016/S0076-6879(67)11064-1
Stark GR, Stein WH, Moore S (1961) Relationships between the conformation of ribonuclease and its reactivity toward iodoacetate. J Biol Chem 236:436–442
Kolb HC, Sharpless KB (2003) The growing impact of click chemistry on drug discovery. Drug Discov Today 8(24):1128–1137. https://doi.org/10.1016/S1359-6446(03)02933-7
Pressly ED, Amir RJ, Hawker CJ (2011) Rapid synthesis of block and cyclic copolymers via click chemistry in the presence of copper nanoparticles. J Polym Sci A Polym Chem 49(3):814–819. https://doi.org/10.1002/pola.24504
Lutz JF, Zarafshani Z (2008) Efficient construction of therapeutics, bioconjugates, biomaterials and bioactive surfaces using azide-alkyne “click” chemistry. Adv Drug Deliv Rev 60:958–970. https://doi.org/10.1016/j.addr.2008.02.004
Guis C, Cheradame H (2000) Synthesis of polymers containing pseudohalide groups by cationic polymerization 15. Study of the functionalizing living cationic polymerization of 2-methyl-2-oxazoline in the presence of trimethylsilylazide. Eur Polym J 36:2581–2590. https://doi.org/10.1016/S0014-3057(00)00071-9
Hoogenboom R, Fijten MWM, Meier MAR, Schubert US (2003) Living cationic polymerizations utilizing an automated synthesizer: high-throughput synthesis of polyoxazolines. Macromol Rapid Commun 24:92–97. https://doi.org/10.1002/marc.200390003
Park JS, Akiyama Y, Winnik FM, Kataoka K (2004) Versatile synthesis of end-functionalized thermosensitive poly(2-isopropyl-2-oxazolines). Macromolecules 37:6786–6792. https://doi.org/10.1021/ma049677n
Aoi K, Okada M (1996) Polymerization of oxazolines. Prog Polym Sci 21:151–208. https://doi.org/10.1016/0079-6700(95)00020-8
Hoogenboom R, Fijten MWM, Schubert US (2004) Parallel kinetic investigation of 2-oxazoline polymerizations with different initiators as basis for designed copolymer synthesis. J Polym Sci A Polym Chem 42:1830–1840. https://doi.org/10.1002/pola.20024
Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM (1999) Polymeric systems for controlled drug release. Chem Rev 99:3181–3198. https://doi.org/10.1021/cr940351u
Jeong B, Bae YH, Lee DS, Kim SW (1997) Biodegradable block copolymers as injectable drug-delivery systems. Nature 388:860–862. https://doi.org/10.1038/42218
Kowalski A, Duda A, Pencaek S (2000) Mechanism of cyclic ester polymerization initiated with tin (II) octoate. 2. Macromolecules fitted with tin(II) alkoxide species observed directly in MALDI-TOF spectra. Macromolecules 33:689–695. https://doi.org/10.1021/ma9906940
Alvaradejo GG, Glassner M, Hoogenboom R, Delaittre G (2018) Maleimide end-functionalized poly(2-oxazoline)s by the functional initiator route: synthesis and (bio)conjugation. RSC Adv 8:9471–9479. https://doi.org/10.1039/C8RA00948A
Bontempo D, Heredia KL, Fish BA, Maynard HD (2004) Cysteine-reactive polymers synthesized by atom transfer radical polymerization for conjugation to proteins. J Am Chem Soc 126:15372–15373. https://doi.org/10.1021/ja045063m
Mathews AS, Ahmed S, Shahin M, Lavasanifar A, Kaur K (2013) Peptide modified polymeric micelles specific for breast cancer cells. Bioconj Chem 24:560–570. https://doi.org/10.1021/bc3004364
Etayash H, Jiang K, Azmi S, Thundat T, Kaur K (2015) Real-time detection of breast cancer cells using peptide functionalized microcantilever arrays. Sci Rep 5(13967):1–13. https://doi.org/10.1038/srep13967
Lewandowski B, de Bo G, Ward JW, Papmeyer M, Kuschel S, Aldegunde MJ, Gramlich PME, Heckmann D, Goldup SM, D’Souza DM, Fernandes AE, Leigh DA (2013) Sequence-specific peptide synthesis by an artificial small-molecule machine. Science 339(6116):189–193. https://doi.org/10.1126/science.1229753
Acknowledgements
The authors would like to thank Turkish Scientific and Technological Council (TUBITAK-213M725) for financial supports.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ozkose, U.U., Yilmaz, O. & Alpturk, O. Synthesis of poly(2-ethyl-2-oxazoline)-b-poly(ε-caprolactone) conjugates by a new modular strategy. Polym. Bull. 77, 5647–5662 (2020). https://doi.org/10.1007/s00289-019-03038-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-03038-w