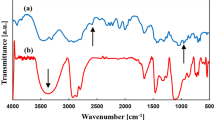
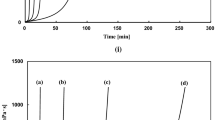
Abstract
Gels containing acetal group have been synthesized by addition reaction of multi-functional phenols, 1,1,1-tris(4-hydroxyphenyl)ethane (THPE) or tannic acid (TA) and divinylethers, diethylene glycol divinyl ether (DEGVE) or polyethylene glycol divinyl ether (PEGVE) in tetrahydrofuran (THF) or 1,4-dioxane (DO) using pyridinium p-toluenesulfonate as a catalyst under nitrogen atmosphere. The gels synthesized from DEGVE showed higher Young’s modulus, breaking stress, and lower breaking strain than the gels synthesized from PEGVE. The gels in DO showed higher mechanical properties than those in THF due to the high affinity between the network structure and the solvent used. The gels with TA showed lower Young’s modulus than those with THPE derived from flexible molecular structure of TA. The reaction of THPE and PEGVE in acetonitrile induced phase separation, and yielded porous polymer formed by connected globules about 10 μm diameter. The dried porous polymers showed remarkable increase in the Young’s modulus in comparison with the corresponding gels in THF or DO. The gels and porous polymers were degraded under atmospheric conditions caused by hydrolytic degradation of acetal groups in the network structure. The present hydrolytic degradable materials would be applicable for drug carriers or sensors for humidity or water.









Similar content being viewed by others
References
Garrison T, Murawski A, Quirino R (2016) Bio-based polymers with potential for biodegradability. Polymers 8:262. https://doi.org/10.3390/polym8070262
Acik G, Karabulut HRF, Altinkok C, Karatavuk AO (2019) Synthesis and characterization of biodegradable polyurethanes made from cholic and L-lysine diisocyanate ethyl ether. Polym Degrad Stab 165:43–48. https://doi.org/10.1016/j.polymdegradstab.2019.04.015
Kenley RA, Manser GE (1985) Degradable polymers. Incorporating a difunctional azo compound into a polymer network to produce thermally degradable polyurethanes. Macromolecules 18:127–131. https://doi.org/10.1021/ma00144a002
Ogino K, Chen J-S, Ober CK (1988) Synthesis and characterization of thermally degradable polymer networks. Chem Mater 10:383–3838. https://doi.org/10.1021/cm9801183
Burkoth AK, Anseth KS (1991) MALD-TOF characterization of highly cross-linked, degradable polymer networks. Macromolecules 32:1438–1444. https://doi.org/10.1021/ma9814651
Timmer MD, Jo S, Wang C, Ambrose CG, Mikos AG (2002) Characterization of the cross-linked structure of fumarate-based degradable polymer networks. Macromolecules 35:4373–4379. https://doi.org/10.1021/ma020028q
Chen JS, Ober CK, Poliks MD (2002) Characterization of thermally reworkable thermosets: materials for environmentally friendly processing and reuse. Polymer 43:131–139. https://doi.org/10.1016/S0032-3861(01)00605-X
Shirai M, Morishita S, Okamura H, Tsunooka M (2002) Photo-cross-linkable polymers with thermally degradable property. Chem Mater 14:334–340. https://doi.org/10.1021/cm0103646
Johnson JA, Lewis DR, Diaz DD, Finn MG, Koberstein JT, Turro NJ (2006) Synthesis of degradable model networks via ATRP and click chemistry. J Am Chem Soc 128:6564–6565. https://doi.org/10.1021/ja0612910
Matsukawa D, Okamura H, Shirai M (2007) Degradable network polymers based on di(metha)acrylate. Chem Lett 36:1290–1291. https://doi.org/10.1246/cl.2007.1290
Kitamura T, Matsumoto A (2007) Facile synthesis of degradable gels by oxygen cross-linking of polymers including a dienyl group on their side chain or at chain ends. Macromolecules 40:6143–6149. https://doi.org/10.1021/ma0707537
Kitamura T, Matsumoto A (2007) Synthesis of poly(lactic acid) with branched and network structures containing thermally degradable junctions. Macromolecules 40:509–517. https://doi.org/10.1021/ma0621829
Brauer DS, Russel C, Vogt S, Weisser J, Schnabelrauch M (2008) Degradable phosphate glass fiber reinforced polymer matrices: mechanical properties and cell response. J Mater Sci Mater Med 19:121–127. https://doi.org/10.1007/s10856-007-3147-x
Shipp DA, McQuinn CW, Rutherglen BG, McBath RA (2009) Elastomeric and degradable polyanhydride network polymers by step-growth thiol-ene photopolymerization. Chem Commun 1:6415–6417. https://doi.org/10.1039/B911557A
Mihashi A, Tamura H, Sato H, Matsumoto A (2010) Synthesis of degradable network polymers containing peroxy units in the main chain or the cross-linking point. Prog Org Coat 68:42–47. https://doi.org/10.1016/j.porgcoat.2009.10.008
Safranski DL, Crabtree JC, Huq YR, Gall K (2011) Thermo-mechanical properties of semi-degradable poly(β-amino ester)-co-methyl methacrylate networks under simulated physiological conditions. Polymer 52:4920–4927. https://doi.org/10.1016/j.polymer.2011.08.033
Fukuda K, Shimoda M, Sukegawa M, Nobori T, Lehn JM (2012) Doubly degradable dynamers: dynamic covalent polymers based on reversible imine and biodegradable polyester units. Green Chem 14:2907–2911. https://doi.org/10.1039/c2gc35875a
Kharkar RM, Kiick KL, Kloxin AM (2013) Designing degradable hydrogels for orthogonal control of cell microenvironment. Chem Soc Rev 42:7355–7372. https://doi.org/10.1039/c3cs60040h
Shirai M (2014) Photocrosslinkable polymers with degradable properties. Polym J 46:859–865. https://doi.org/10.1038/pj.2014.79
Ware T, Jennings AR, Bassampour ZS, Simon D, Son DY, Voit W (2014) Degradable, silyl ether thiol-ene networks. RSC Adv 4:39991–40002. https://doi.org/10.1039/C4RA06997H
Kharkar PM, Kiick K, Kloxin AP (2015) Design of thiol- and light-sensitive degradable hydrogels using Michael-type addition reactions. Polym Chem 6:5565–5574. https://doi.org/10.1039/c5py00750j
Xu F, Sheardown H, Hoare T (2016) Reactive electrospinning of degradable poly(oligoethylene glycol methacrylate)-based nanofibrous hydrogel network. Chem Commun 52:1451–1454. https://doi.org/10.1039/c5cc08053c
Kamaly N, Yameen B, Wu J, Farokhzad OC (2016) Degradable controlled-release polymers and polymeric nanoparticles: mechanics of controlling drug release. Chem Rev 116:2602–2663. https://doi.org/10.1021/acs.chemrev.5b00346
Lu W, Pan X, Zhang Z, Zhu J, Zhou N, Zhu X (2017) A degradable cross-linked polymer containing dynamic covalent selenide bond. Polym Chem 8:3874–3880. https://doi.org/10.1039/c37py00719a
Babra TS, Trivedi A, Warriner CN, Bazin N, Castiglione D, Sivour C, Hayes W, Greenland BW (2017) Fluoride degradable and thermally debondable polyurethane based adhesive. Polym Chem 8:7207–7216. https://doi.org/10.1039/c7py01653k
Bednarek M, Kubisa P (2019) Reversible networks of degradable polyesters containing weak covalent bonds. Polym Chem 10:1848–1872. https://doi.org/10.1039/c8py01731j
De Clercq RR, Goethals EJ (1992) Polymer networks containing degradable polyacetal segments. Macromolecules 25:1109–1113. https://doi.org/10.1021/ma00029a016
Buchwalter SL, Kosbar LL (1996) Cleavable epoxy resins: design for disassembly of a thermoset. J Polym Sci Part A Polym Chem 34:249–260. https://doi.org/10.1002/(SICI)1099-0518(19960130)
Sui XC, Shi Y, Fu ZF (2010) Novel degradable polymer networks containing acetal components and well-defined backbones. Australian J Chem 63:1497–1501. https://doi.org/10.1071/CH10207
Sui X (2011) Shi Y, Fu Z (2011) Novel degradable polymer networks containing acetal components. Sci China Chem 54:419–425. https://doi.org/10.1007/s11426-011-4223-0
Kepola EJ, Patrickios CS (2018) Networks based on “core-first” star polymers end-linked using a degradable ketal cross-linker: synthesis characterization, and cleavage. Macromol Chem Phys 219:1700404. https://doi.org/10.1002/macp.201700404
Rikkou-Kalourkoti M, Loizou E, Porcar L, Matyjaszewski K, Patrickios CS (2012) End-linked, amphiphilic, degradable polymer conetworks: synthesis by sequential atom transfer radical polymerization using a bifunctional, cleavable initiator. Polym Chem 3:105–116. https://doi.org/10.1039/c1py00349f
Zhao C, Patel K, Aichinger LM, Liu Z, Hu R, Chen H, Li X, Li L, Zhang G, Chang Y, Zheng J (2013) Antifouling and biodegradable poly(N-hydroxyethyl acrylamide) (polyHEAA)-based nanogels. RSC Adv 3:19991–20000. https://doi.org/10.1039/c3ra42323a
Cao H, Dong Y, Bre L, Tapeinos C, Wang W, Pandit A (2016) An acetal-based polymeric crosslinker with controlled pH-sensitivity. RSC Adv 6:9064–9611. https://doi.org/10.1039/c6ra00423g
Amato DV, Amato DN, Blancett LT, Mavrodi OV, Martin WB, Swilley SN, Sandoz MJ, Shearer G, Mavrodi DV, Patton DL (2018) A bio-based pro-antimicrobial polymer network via degradable acetal linkages. Acta Biomater 67:196–205. https://doi.org/10.1016/j.actbio.2017.12.016
Miller KA, Morado EG, Samanta SR, Walker BA, Nelson AZ, Sen S, Tran DT, Whitaker DJ, Ewoldt RH, Braun PV, Zimmerman SC (2019) Acid-triggered, acid-generating, and self-amplifying degradable polymers. J Am Chem Soc 141:2838–2842. https://doi.org/10.1021/jacs.8b07705
Naga N, Oda E, Toyota A, Horie K, Furukawa H (2006) Tailored synthesis and fundamental characterization of organic-inorganic hybrid gels by means of a hydrosilylation reaction. Macromol Chem Phys 207:627–635. https://doi.org/10.1002/macp.200500501
Naga N, Oda E, Toyota A, Furukawa H (2007) Mesh size control of organic-inorganic hybrid gels by means of a hydrosilylation co-gelation of siloxane or silsesquioxane and α, ω-non-conjugated dienes. Macromol Chem Phys 208:2331–2338. https://doi.org/10.1002/macp.200700184
Naga N, Kihara Y, Miyanaga T, Furukawa H (2009) Synthesis of organic−inorganic hybrid gels from siloxane or silsesquioxane and α, ω-nonconjugated dienes by means of a photo hydrosilylation reaction. Macromolecules 42:3454–3462https://doi.org/10.1021/ma802745x
Naga N, Nagino H, Furukawa H (2016) Synthesis of organic-inorganic hybrid gels by means of thiol-ene and azide-alkene reactions. J Polym Sci Part A Polym Chem 54:2229–2238. https://doi.org/10.1002/pola.28096
Naga N, Michida R, Kudo S, Nagami Y, Moriyama K, Nageh H, Furukawa H, Nakano T (2019) Synthesis of joint-linker type gels and porous polymers by addition reactions of multi-functional thiol and alkyl diacrylate, diisocyanate compounds. Mater Today Commun 1:18153–18162. https://doi.org/10.1016/j.mtcomm.2018.11.013
Hashimoto T, Misawa K, Urushisaki M (2008) Synthesis and properties of chemically recyclable polyurethane thermoplastic elastomers containing degradable polyacetal soft segments. Kobunshi Ronbunshu 65:178–184. https://doi.org/10.1295/koron.65.178
Hashimoto T, Umehara A, Ishizuka K, Kodaira T (2001) New synthetic method of hydroxyl-terminated telechelic polyacetals based on polyaddition of hydroxyalkyl vinyl ether in the presence of diol. Proc Jpn Acad Ser B 77:63–67. https://doi.org/10.2183/pjab.77.63
Hashimoto T, Ishizuka K, Umehara A, Kodaira T (2002) Synthesis of polyacetals with various main-chain structures by the self-polyaddition of vinyl ethers with a hydroxyl function. J Polym Sci Part A Polym Chem 40:4053–4064. https://doi.org/10.1002/pola.10490
Hashimoto T, Umehara A, Urushisaki M, Kodaira T (2004) Synthesis of a new degradable polyurethane elastomer containing polyacetal soft segments. J Polym Sci Part A Polym Chem 42:2766–2773. https://doi.org/10.1002/pola.20138
Fedros RF (1974) A method for estimating both the solubility parameters and molar volumes of liquids. Polym Eng Sci 14:147–154. https://doi.org/10.1002/pen.760140211
Inoue T (1995) Reaction-induced phase decomposition in polymer blends. Prog Polym Sci 20:119–153. https://doi.org/10.1016/0079-6700(94)00032-W
Yamanaka K, Inoue T (1989) Structure development in epoxy resin modified with poly(ether sulphone). Polymer 30:662–667.https://doi.org/10.1016/0032-3861(89)90151-1
Ohnaga T, Inoue T (1989) Growth and decay of concentration fluctuations in polymer–polymer mixtures. J Polym Sci Part B Polym Phys 27:1675–1689. https://doi.org/10.1002/polb.1989.090270807
Inoue T, Ichihara S (1988) Polymer alloy. In: Society of Polymer Science (ed). Kyoritsu Shuppan, Japan
Acknowledgement
This work was partially supported by JSPS KAKENHI Grant Number 24550261.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Naga, N., Hasegawa, K., Nageh, H. et al. Synthesis and properties of degradable gels and porous polymers including acetal group in the network structure by addition reaction of multi-functional phenols and divinyl ether compounds. Polym. Bull. 77, 5631–5645 (2020). https://doi.org/10.1007/s00289-019-03033-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-03033-1