Abstract
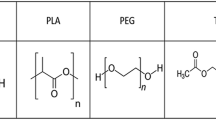
The objective of this study was to investigate and compare the effects of two different types of polyols on properties of synthesized polyurethanes (PUs), to develop biomedical applications of these materials. Polyurethane membranes were prepared from two different polyols (castor oil and CAPA 7201) as wound healing films. The chemical, physical, mechanical and biological properties of the prepared membranes were studied. Both films had smooth surfaces, with no signs of pores and cracks. CAPA-based polyurethane showed higher crystallinity and lower thermal stability compared to the other polyurethanes. Results of contact angle and water vapor transmission rate (WVTR) showed no significant differences between two different polyols used in PU synthesis. The water absorption of the CAPA-based PU (5.67%) was higher than the castor oil-based PU (0.76%) after immersion in water for 3 days at 37 °C. The values of WVTR were obtained as 260 and 285 g/m2/day for PCL and castor oil-based PUs, respectively. CAPA-based PU membrane showed 4 MPa tensile strength and about 550% elongation at break which is higher than the other samples (1.7 MPa and 100%). The viability of L-929 mouse cell line in contact with CAPA-based PU film was greater than 80% and showed a lack of toxicity of the synthesized polymer. The in vivo wound healing model evaluation using a full-thickness rat model experiment was carried out within 13 days. The wound size reduction of castor oil and CAPA-based PUs reached to 99% compared to 68% in gauze sample on the 13th day after surgery. The in vivo results revealed that PUs derived from CAPA 7201 was milder and led to less inflammatory response compared to castor oil which consequently was completely filled with new epithelium without any significant adverse reactions. Consequently, results confirmed the potential use of CAPA-based PU in biomedical field.







Similar content being viewed by others
References
Zhong S, Zhang Y, Lim C (2010) Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip Rev: Nanomed Nanobiotechnol 2(5):510–525
Paul W, Sharma CP (2004) Chitosan and alginate wound dressings: a short review. Trends Biomater Artif Organs 18(1):18–23
Zhang Z, Michniak-Kohn BB (2012) Tissue engineered human skin equivalents. Pharmaceutics 4(1):26–41
Groeber F, Holeiter M, Hampel M, Hinderer S, Schenke-Layland K (2011) Skin tissue engineering—in vivo and in vitro applications. Adv Drug Deliv Rev 63(4–5):352–366
Kaur T, Thirugnanam A (2017) Effect of porous activated charcoal reinforcement on mechanical and in-vitro biological properties of polyvinyl alcohol composite scaffolds. J Mater Sci Technol 33(7):734–743. https://doi.org/10.1016/j.jmst.2016.06.020
Khalili S, Nouri Khorasani S, Razavi M, Hashemi Beni B, Heydari F, Tamayol A (2018) Nanofibrous scaffolds with biomimetic structure. J Biomed Mater Res, Part A 106(2):370–376. https://doi.org/10.1002/jbm.a.36246
Khalili S, Khorasani SN, Razavi SM, Hashemibeni B, Tamayol A (2019) Nanofibrous scaffolds with biomimetic composition for skin regeneration. Appl Biochem Biotechnol 187(4):1193–1203. https://doi.org/10.1007/s12010-018-2871-7
Rawlings AV, Harding CR (2004) Moisturization and skin barrier function. Dermatol Ther 17(s1):43–48
MacNeil S (2007) Progress and opportunities for tissue-engineered skin. Nature 445(7130):874
Zaidi Z, Lanigan SW (2010) Dermatology in clinical practice. Springer, Berlin
Yari A, Yeganeh H, Bakhshi H, Gharibi R (2014) Preparation and characterization of novel antibacterial castor oil-based polyurethane membranes for wound dressing application. J Biomed Mater Res, Part A 102(1):84–96
Zhen Z, Zheng Y, Ge Z, Lai C, Xi T (2017) Biological effect and molecular mechanism study of biomaterials based on proteomic research. J Mater Sci Technol 33(7):607–615. https://doi.org/10.1016/j.jmst.2017.01.001
Rezvani Ghomi E, Khalili S, Nouri Khorasani S, Esmaeely Neisiany R, Ramakrishna S (2019) Wound dressings: current advances and future directions. J Appl Polym Sci 136(27):47738. https://doi.org/10.1002/app.47738
Kozen BG, Kircher SJ, Henao J, Godinez FS, Johnson AS (2008) An alternative hemostatic dressing: comparison of CELOX, HemCon, and QuikClot. Acad Emerg Med 15(1):74–81
Ong S-Y, Wu J, Moochhala SM, Tan M-H, Lu J (2008) Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 29(32):4323–4332
Kumar PS, Abhilash S, Manzoor K, Nair S, Tamura H, Jayakumar R (2010) Preparation and characterization of novel β-chitin/nanosilver composite scaffolds for wound dressing applications. Carbohydr Polym 80(3):761–767
Metcalfe AD, Ferguson MW (2007) Bioengineering skin using mechanisms of regeneration and repair. Biomaterials 28(34):5100–5113
Hein S, Wang K, Stevens W, Kjems J (2008) Chitosan composites for biomedical applications: status, challenges and perspectives. Mater Sci Technol 24(9):1053–1061
Kumar MNR (2000) A review of chitin and chitosan applications. React Funct Polym 46(1):1–27
Jung YC, Cho JW (2010) Application of shape memory polyurethane in orthodontic. J Mater Sci: Mater Med 21(10):2881–2886
Yari A, Yeganeh H, Bakhshi H (2012) Synthesis and evaluation of novel absorptive and antibacterial polyurethane membranes as wound dressing. J Mater Sci: Mater Med 23(9):2187–2202
Bakhshi H, Yeganeh H, Yari A, Nezhad SK (2014) Castor oil-based polyurethane coatings containing benzyl triethanol ammonium chloride: synthesis, characterization, and biological properties. J Mater Sci 49(15):5365–5377
Moghanizadeh-Ashkezari M, Shokrollahi P, Zandi M, Shokrolahi F (2018) Polyurethanes with separately tunable biodegradation behavior and mechanical properties for tissue engineering. Polym Adv Technol 29(1):528–540. https://doi.org/10.1002/pat.4160
Amina M, Amna T, Hassan MS, Ibrahim TA, Khil M-S (2013) Facile single mode electrospinning way for fabrication of natural product based silver decorated polyurethane nanofibrous membranes: prospective medicated bandages. Colloids Surf, A 425:115–121
Lu Q-W, Macosko CW (2004) Comparing the compatibility of various functionalized polypropylenes with thermoplastic polyurethane (TPU). Polymer 45(6):1981–1991
Cao X, Lee LJ, Widya T, Macosko C (2005) Polyurethane/clay nanocomposites foams: processing, structure and properties. Polymer 46(3):775–783
Yeganeh H, Lakouraj MM, Jamshidi S (2005) Synthesis and characterization of novel biodegradable epoxy-modified polyurethane elastomers. J Polym Sci, Part A: Polym Chem 43(14):2985–2996
Xu R, Luo G, Xia H, He W, Zhao J, Liu B, Tan J, Zhou J, Liu D, Wang Y (2015) Novel bilayer wound dressing composed of silicone rubber with particular micropores enhanced wound re-epithelialization and contraction. Biomaterials 40:1–11
Hasirci N, Aksoy EA (2007) Synthesis and modifications of polyurethanes for biomedical purposes. High Perform Polym 19(5–6):621–637
Pradhan KC, Nayak P (2012) Synthesis and characterization of polyurethane nanocomposite from castor oil-hexamethylene diisocyanate (HMDI). Adv Appl Sci Res 3(5):3045–3052
Rojek P, Prociak A (2012) Effect of different rapeseed-oil-based polyols on mechanical properties of flexible polyurethane foams. J Appl Polym Sci 125(4):2936–2945
Ronda JC, Lligadas G, Galià M, Cádiz V (2011) Vegetable oils as platform chemicals for polymer synthesis. Eur J Lipid Sci Technol 113(1):46–58
Lligadas G, Ronda JC, Galia M, Cadiz V (2013) Renewable polymeric materials from vegetable oils: a perspective. Mater Today 16(9):337–343
Bakhshi H, Yeganeh H, Mehdipour-Ataei S, Shokrgozar MA, Yari A, Saeedi-Eslami SN (2013) Synthesis and characterization of antibacterial polyurethane coatings from quaternary ammonium salts functionalized soybean oil based polyols. Mater Sci Eng, C 33(1):153–164
Das S, Pandey P, Mohanty S, Nayak SK (2017) Insight on castor oil based polyurethane and nanocomposites: recent trends and development. Polym-Plast Technol Eng 56(14):1556–1585
Yeganeh H, Hojati-Talemi P (2007) Preparation and properties of novel biodegradable polyurethane networks based on castor oil and poly (ethylene glycol). Polym Degrad Stab 92(3):480–489
Cangemi JM, Claro Neto S, Chierice GO, Santos AMd (2006) Study of the biodegradation of a polymer derived from castor oil by scanning electron microscopy, thermogravimetry and infrared spectroscopy. Polímeros 16(2):129–135
Cangemi JM, Santos AMd, Claro Neto S, Chierice GO (2008) Biodegradation of polyurethane derived from castor oil. Polímeros 18(3):201–206
Sponton M, Casis N, Mazo P, Raud B, Simonetta A, Ríos L, Estenoz D (2013) Biodegradation study by pseudomonas sp. of flexible polyurethane foams derived from castor oil. Int Biodeterior Biodegrad 85:85–94
McBane JE, Sharifpoor S, Cai K, Labow RS, Santerre JP (2011) Biodegradation and in vivo biocompatibility of a degradable, polar/hydrophobic/ionic polyurethane for tissue engineering applications. Biomaterials 32(26):6034–6044
Han J, Chen B, Ye L, Zhang A-y, Zhang J, Feng Z-g (2009) Synthesis and characterization of biodegradable polyurethane based on poly (ε-caprolactone) and l-lysine ethyl ester diisocyanate. Front Mater Sci Chin 3(1):25–32
Vatankhah E, Semnani D, Prabhakaran MP, Tadayon M, Razavi S, Ramakrishna S (2014) Artificial neural network for modeling the elastic modulus of electrospun polycaprolactone/gelatin scaffolds. Acta Biomater 10(2):709–721. https://doi.org/10.1016/j.actbio.2013.09.015
da Silva GR, da Silva-Cunha Jr A, Behar-Cohen F, Ayres E, Oréfice RL (2010) Biodegradation of polyurethanes and nanocomposites to non-cytotoxic degradation products. Polym Degrad Stab 95(4):491–499
Uscátegui YL, Arévalo FR, Díaz LE, Cobo MI, Valero MF (2016) Microbial degradation, cytotoxicity and antibacterial activity of polyurethanes based on modified castor oil and polycaprolactone. J Biomater Sci Polym Ed 27(18):1860–1879
Liu X, Niu Y, Chen KC, Chen S (2017) Rapid hemostatic and mild polyurethane-urea foam wound dressing for promoting wound healing. Mater Sci Eng, C 71:289–297
Siafaka PI, Zisi AP, Exindari MK, Karantas ID, Bikiaris DN (2016) Porous dressings of modified chitosan with poly (2-hydroxyethyl acrylate) for topical wound delivery of levofloxacin. Carbohydr Polym 143:90–99
Atar-Froyman L, Sharon A, Weiss EI, Houri-Haddad Y, Kesler-Shvero D, Domb AJ, Pilo R, Beyth N (2015) Anti-biofilm properties of wound dressing incorporating nonrelease polycationic antimicrobials. Biomaterials 46:141–148
Balakrishnan B, Mohanty M, Fernandez AC, Mohanan PV, Jayakrishnan A (2006) Evaluation of the effect of incorporation of dibutyryl cyclic adenosine monophosphate in an in situ-forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 27(8):1355–1361
Yates CC, Whaley D, Babu R, Zhang J, Krishna P, Beckman E, Pasculle AW, Wells A (2007) The effect of multifunctional polymer-based gels on wound healing in full thickness bacteria-contaminated mouse skin wound models. Biomaterials 28(27):3977–3986
Barikani M, Zia KM, Bhatti IA, Zuber M, Bhatti HN (2008) Molecular engineering and properties of chitin based shape memory polyurethanes. Carbohydr Polym 74(3):621–626. https://doi.org/10.1016/j.carbpol.2008.04.023
Cervantes-Uc JM, Cauich-Rodríguez JV, Vázquez-Torres H, Garfias-Mesías LF, Paul DR (2007) Thermal degradation of commercially available organoclays studied by TGA–FTIR. Thermochim Acta 457(1):92–102. https://doi.org/10.1016/j.tca.2007.03.008
Cervantes-Uc JM, Espinosa JIM, Cauich-Rodríguez JV, Ávila-Ortega A, Vázquez-Torres H, Marcos-Fernández A, San Román J (2009) TGA/FTIR studies of segmented aliphatic polyurethanes and their nanocomposites prepared with commercial montmorillonites. Polym Degrad Stab 94(10):1666–1677. https://doi.org/10.1016/j.polymdegradstab.2009.06.022
Sánchez-Adsuar MS (2000) Influence of the composition on the crystallinity and adhesion properties of thermoplastic polyurethane elastomers. Int J Adhes Adhes 20(4):291–298. https://doi.org/10.1016/S0143-7496(99)00059-7
Heijkants RGJC, Calck RVv, van Tienen TG, de Groot JH, Buma P, Pennings AJ, Veth RPH, Schouten AJ (2005) Uncatalyzed synthesis, thermal and mechanical properties of polyurethanes based on poly(ε-caprolactone) and 1,4-butane diisocyanate with uniform hard segment. Biomaterials 26(20):4219–4228. https://doi.org/10.1016/j.biomaterials.2004.11.005
Voda A, Beck K, Schauber T, Adler M, Dabisch T, Bescher M, Viol M, Demco DE, Blümich B (2006) Investigation of soft segments of thermoplastic polyurethane by NMR, differential scanning calorimetry and rebound resilience. Polym Test 25(2):203–213. https://doi.org/10.1016/j.polymertesting.2005.10.007
Bagdi K, Molnár K, Wacha A, Bóta A, Pukánszky B (2011) Hierarchical structure of phase-separated segmented polyurethane elastomers and its effect on properties. Polym Int 60(4):529–536. https://doi.org/10.1002/pi.3003
Kloss J, Munaro M, De Souza GP, Gulmine JV, Wang SH, Zawadzki S, Akcelrud L (2002) Poly (ester urethane) s with polycaprolactone soft segments: a morphological study. J Polym Sci, Part A: Polym Chem 40(23):4117–4130
Valério A, Conti DS, Araújo PHH, Sayer C, Rocha SRPd (2015) Synthesis of PEG-PCL-based polyurethane nanoparticles by miniemulsion polymerization. Colloids Surf, B 135:35–41. https://doi.org/10.1016/j.colsurfb.2015.07.044
Coutinho FM, Delpech MC (2000) Degradation profile of films cast from aqueous polyurethane dispersions. Polym Degrad Stab 70(1):49–57
Javni I, Petrović ZS, Guo A, Fuller R (2000) Thermal stability of polyurethanes based on vegetable oils. J Appl Polym Sci 77(8):1723–1734
Saadatkish N, Nouri Khorasani S, Morshed M, Allafchian A-R, Beigi M-H, Masoudi Rad M, Esmaeely Neisiany R, Nasr-Esfahani M-H (2018) A ternary nanofibrous scaffold potential for central nerve system tissue engineering. J Biomed Mater Res, Part A 106(9):2394–2401. https://doi.org/10.1002/jbm.a.36431
Yilgör E, Isik M, Söz CK, Yilgör I (2016) Synthesis and structure-property behavior of polycaprolactone-polydimethylsiloxane-polycaprolactone triblock copolymers. Polymer 83:138–153
Wu G-H, Hsu S-h (2016) Synthesis of water-based cationic polyurethane for antibacterial and gene delivery applications. Colloids Surf, B 146:825–832
Hill MJ, Cheah C, Sarkar D (2016) Interfacial energetics approach for analysis of endothelial cell and segmental polyurethane interactions. Colloids Surf, B 144:46–56
Abdali Z, Yeganeh H, Solouk A, Gharibi R, Sorayya M (2015) Thermoresponsive antimicrobial wound dressings via simultaneous thiol-ene polymerization and in situ generation of silver nanoparticles. RSC Adv 5(81):66024–66036
Sathiskumar P, Chopra S, Madras G (2012) Investigation of biodegradable and biocompatible castor oil poly (mannitol-citric-sebacate) polyester as a drug carrier. Curr Sci 102:97–104
Bishop S, Walker M, Rogers A, Chen W (2003) Importance of moisture balance at the wound-dressing interface. J Wound Care 12(4):125–128
Hess CT, Kirsner RS (2003) Orchestrating wound healing: assessing and preparing the wound bed. Adv Skin Wound Care 16(5):246–257
Wu P, Fisher A, Foo P, Queen D, Gaylor J (1995) In vitro assessment of water vapour transmission of synthetic wound dressings. Biomaterials 16(3):171–175
Seaman S (2002) Dressing selection in chronic wound management. J Am Podiatr Med Assoc 92(1):24–33
Lamke L-O, Nilsson G, Reithner H (1977) The evaporative water loss from burns and the water-vapour permeability of grafts and artificial membranes used in the treatment of burns. Burns 3(3):159–165
Yoo HJ, Kim HD (2008) Characteristics of waterborne polyurethane/poly (N-vinylpyrrolidone) composite films for wound-healing dressings. J Appl Polym Sci 107(1):331–338
Guan J, Sacks MS, Beckman EJ, Wagner WR (2004) Biodegradable poly (ether ester urethane) urea elastomers based on poly (ether ester) triblock copolymers and putrescine: synthesis, characterization and cytocompatibility. Biomaterials 25(1):85–96
Lee SM, Park IK, Kim YS, Kim HJ, Moon H, Mueller S, Jeong Y-I (2016) Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomater Res 20:15. https://doi.org/10.1186/s40824-016-0063-5
Das S, Pandey P, Mohanty S, Nayak SK (2017) Insight on castor oil based polyurethane and nanocomposites: recent trends and development. Polym-Plast Technol Eng 56:1–30
Reddy TT, Kano A, Maruyama A, Takahara A (2010) Synthesis, characterization and drug release of biocompatible/biodegradable non-toxic poly (urethane urea) s based on poly (ε-caprolactone) s and lysine-based diisocyanate. J Biomater Sci Polym Ed 21(11):1483–1502
Zia KM, Zuber M, Bhatti IA, Barikani M, Sheikh MA (2009) Evaluation of biocompatibility and mechanical behavior of polyurethane elastomers based on chitin/1, 4-butane diol blends. Int J Biol Macromol 44(1):18–22
Acknowledgements
This work was supported by the Iranian National Elites Foundation under contract 5972. The authors would like to appreciate financial support. This study was approved by the medical ethics and research office at the Isfahan University of Medical Sciences, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rezaei Hosseinabadi, S., Parsapour, A., Nouri Khorasani, S. et al. Wound dressing application of castor oil- and CAPA-based polyurethane membranes. Polym. Bull. 77, 2945–2964 (2020). https://doi.org/10.1007/s00289-019-02891-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02891-z