Abstract
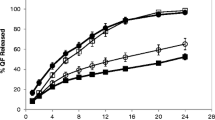
This work is based on formulating and optimizing controlled release (CR) valsartan (160 mg) tablets using different viscosity grades of the cellulosic polymer. The objective was to develop an effective once-daily drug delivery system of this cardiovascular agent. Central composite design was used for designing the formulations. Polymers used were Methocel® K4M, K15M and K100M. Compatibility of excipients with active was studied through FT-IR. Micromeritic properties were determined and formulations exhibiting appropriate flow characteristics were compressed. Swelling behavior and in vitro buoyancy effect were studied and response surface curves were constructed to optimize the formulation. Multi-point dissolution profiles of valsartan CR tablets at pH 1.2, 4.5 and 6.8 were obtained. Model-dependent and model-independent methods were performed including f2, stability test as per ICH guidelines and ANOVA. FT-IR studies revealed the compatibility of valsartan with all excipients. Formulation K4T9 (containing 25% K4M polymer) was selected to be the best optimized trial, based on physical properties and controlled release profile (23% at 4 h, 82% at 16 h and 100% at 24 h). Results of buoyancy and swelling behavior indicated that HPMC-K4M polymer exhibited excellent floating lag time and swelling indexes. In vitro drug release kinetics showed that formulation K4T9 displayed Korsmeyer–Peppas drug release pattern with r value > 0.99. The manufacturing process of K4T9 was also found to be reproducible with a shelf life period of 41 and 36 months at room temperature and accelerated conditions, respectively. Valsartan CR matrix-based formulation was successfully prepared with Methocel K4M retardant.





Similar content being viewed by others
References
Chien Y (1992) Oral drug delivery and delivery systems. In: Chien YW (ed) Novel drug delivery systems. Marcel Dekker, New York
Vyas SP, Khar RK (2002) Controlled drug delivery concepts and advances. Vallabh Prakashan 1:411–447
Shah SU, Shah KU, Rehman A, Khan GM (2011) Investigating the in vitro drug release kinetics from controlled release diclofenac potassium-ethocel matrix tablets and the influence of co-excipients on drug release patterns. Pak J Pharm Sci 24:183–192
Bayomi MA, Al-Suwayeh SA, El-Helw A-RM (2001) Excipient–excipient interaction in the design of sustained-release theophylline tablets: in vitro and in vivo evaluation. Drug Dev Ind Pharm 27(6):499–506
Baveja S, Rao KR, Devi KP (1988) Relationship between gum content and half-life of soluble β blockers from hydrophilic matrix tablets. Int J Pharm 47(1–3):133–139
Colombo P, Bettini R, Santi P, De Ascentiis A, Peppas N (1996) Analysis of the swelling and release mechanisms from drug delivery systems with emphasis on drug solubility and water transport. J Control Release 39(2–3):231–237
Kanakagiri D, Omprakash H, Kumar KM, Vishwanadham Y (2017) Formulation and evaluation of pantoprazole delayed release tablets using Eudragit L30D55. Res J Pharm Technol 10(2):421–425
Pandey SP, Shukla T, Dhote VK, Mishra DK, Maheshwari R, Tekade RK (2019) Use of polymers in controlled release of active agents. In: Basic fundamentals of drug delivery. Elsevier, pp 113–172
Siepmann J, Peppas N (2012) Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev 64:163–174
Chaerunisaa AY, Ali R, Dashevskiy A (2019) Release adjustment of two drugs with different solubility combined in a matrix tablet. AAPS PharmSciTech 20(4):142
Wang S, Wen H, Li P, Cui M, Sun W, Wang H, Liu H, Li S, Pan W, Yang X (2019) Formulation and evaluation of gastric-floating controlled release tablets of Ginkgolides. J Drug Deliv Sci Technol 51:7–17
Rebouh S, Lefnaoui S, Bouhedda M, Yahoum MM, Hanini S (2019) Neuro-fuzzy modeling of ibuprofen-sustained release from tablets based on different cellulose derivatives. Drug Deliv Transl Res 9(1):162–177
Sweetman S (2005) Martindale: the complete drug reference, 34th edn. Pharmaceutical Press, London, pp 900–901
Oparil S, Dyke S, Harris F, Kief J, James D, Hester A, Fitzsimmons S (1996) The efficacy and safety of valsartan compared with placebo in the treatment of patients with essential hypertension. Clin Ther 18(5):797–810
IBM (2019) Micromedex® DRUGDEX® (electronic version). IBM Watson Health, Greenwood Village, Colorado, USA. Available at: https://www.micromedexsolutions.com/
Mahajan P, Mahajan S, Mishra D (2011) Valsartan release from sustained release matrix tablet and effect of cellulose derivatives. Int J Pharm Life Sci 2(1):521–530
Nayak UY, Shavi GV, Nayak Y, Averinen RK, Mutalik S, Reddy SM, Gupta PD, Udupa N (2009) Chronotherapeutic drug delivery for early morning surge in blood pressure: a programmable delivery system. J Control Release 136(2):125–131
Sokar M, Hanafy A, El-Kamel A, El-Gamal S (2013) Pulsatile core-in-cup valsartan tablet formulations: in vitro evaluation. Asian J Pharm Sci 8(4):234–243
Shah S, Patel R, Soniwala M, Chavda J (2015) Development and optimization of press coated tablets of release engineered valsartan for pulsatile delivery. Drug Dev Ind Pharm 41(11):1835–1846
Liltorp K, Larsen TG, Willumsen B, Holm R (2011) Solid state compatibility studies with tablet excipients using non thermal methods. J Pharm Biomed Anal 55(3):424–428
USP35-NF30 U (2012) United States Pharmacopeial. Rockville
Commission USP (2013) USP36/NF31. US Pharmacopeia, Rockville
Cao Q-R, Choi Y-W, Cui J-H, Lee B-J (2005) Effect of solvents on physical properties and release characteristics of monolithic hydroxypropylmethylcellulose matrix granules and tablets. Arch Pharm Res 28(4):493–501
Jiménez-Castellanos MR, Zia H, Rhodes CT (1994) Design and testing in vitro of a bioadhesive and floating drug delivery system for oral application. Int J Pharm 105(1):65–70
Costa P, Lobo JMS (2001) Modeling and comparison of dissolution profiles. Eur J Pharm Sci 13(2):123–133
Shoaib MH, Siddiqi SAS, Yousuf RI, Zaheer K, Hanif M, Rehana S, Jabeen S (2010) Development and evaluation of hydrophilic colloid matrix of famotidine tablets. AAPS PharmSciTech 11(2):708–718
Higuchi WI (1967) Diffusional models useful in biopharmaceutics: drug release rate processes. J Pharm Sci 56(3):315–324
Hixson A, Crowell J (1931) Dependence of reaction velocity upon surface and agitation. Ind Eng Chem 23(8):923–931
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA (1983) Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 15(1):25–35
Langenbucher F (1972) Letters to the editor: linearization of dissolution rate curves by the Weibull distribution. J Pharm Pharmacol 24(12):979–981
Baker RW, Lonsdale HK (1974) Controlled release: mechanisms and rates. In: Tanquary AC, Lacey RE (eds) Controlled release of biologically active agents. Plenum Press, New York, pp 15–71
Guideline IHT (2003) Stability testing of new drug substances and products. Q1A (R2) Curr Step 4:1–24
Hanif M, Shoaib M, Rabia I, Iyad N, Ahmad K, Tariq A (2011) Formulation development and optimization of nimesulide tablets by central composit design and effect of surfactants on dissolution studies. J Pharm Res 4:2447–2452
Chopra S, Patil GV, Motwani SK (2007) Release modulating hydrophilic matrix systems of losartan potassium: optimization of formulation using statistical experimental design. Eur J Pharm Biopharm 66(1):73–82
Cao Q-R, Liu Y, Xu W-J, Lee B-J, Yang M, Cui J-H (2012) Enhanced oral bioavailability of novel mucoadhesive pellets containing valsartan prepared by a dry powder-coating technique. Int J Pharm 434(1–2):325–333
Tiţa B, Fuliaş A, Bandur G, Marian E, Tiţa D (2011) Compatibility study between ketoprofen and pharmaceutical excipients used in solid dosage forms. J Pharm Biomed Anal 56(2):221–227
Verma RK, Garg S (2004) Compatibility studies between isosorbide mononitrate and selected excipients used in the development of extended release formulations. J Pharm Biomed Anal 35(3):449–458
Verma RK, Garg S (2005) Selection of excipients for extended release formulations of glipizide through drug–excipient compatibility testing. J Pharm Biomed Anal 38(4):633–644
Chella N, Tadikonda R (2015) Melt dispersion granules: formulation and evaluation to improve oral delivery of poorly soluble drugs—a case study with valsartan. Drug Dev Ind Pharm 41(6):888–897
Prescott JK, Barnum RA (2000) On powder flowability. Pharm Technol 24(10):60–85
Mendez R, Muzzio F, Velazquez C (2010) Study of the effects of feed frames on powder blend properties during the filling of tablet press dies. Powder Technol 200(3):105–116
Siddiqui A, Nazzal S (2007) Measurement of surface color as an expedient QC method for the detection of deviations in tablet hardness. Int J Pham 341(1–2):173–180
Tan I, Ahmad A, Hameed B (2008) Optimization of preparation conditions for activated carbons from coconut husk using response surface methodology. Chem Eng J 137(3):462–470
Myers RH, Montgomery DC, Anderson-Cook CM (2009) Response surface methodology: process and product optimization using designed experiments (Wiley series in probability and statistics). Wiley, New York
Wu X-G, Li G, Gao Y-L (2006) Optimization of the preparation of nalmefene-loaded sustained-release microspheres using central composite design. Chem Pharm Bull 54(7):977–981
Nellore RV, Rekhi GS, Hussain AS, Tillman LG, Augsburger LL (1998) Development of metoprolol tartrate extended-release matrix tablet formulations for regulatory policy consideration. J Control Release 50(1–3):247–256
Campos-Aldrete ME, Villafuerte-Robles L (1997) Influence of the viscosity grade and the particle size of HPMC on metronidazole release from matrix tablets. Eur J Pharm Biopharm 43(2):173–178
Huang Y-B, Tsai Y-H, Lee S-H, Chang J-S, Wu P-C (2005) Optimization of pH-independent release of nicardipine hydrochloride extended-release matrix tablets using response surface methodology. Int J Pharm 289(1–2):87–95
Mandal U, Gowda V, Ghosh A, Selvan S, Solomon S, Pal TK (2007) Formulation and optimization of sustained release matrix tablet of metformin HCl 500 mg using response surface methodology. Yakugaku Zasshi 127(8):1281–1290
Baumgartner S, Smid-Korbar J, Vrecer F, Kristl J (1998) Physical and technological parameters influencing floating properties of matrix tablets based on cellulose ethers. STP Pharma Sci 8(5):285–290
Wan LSC, Heng PWS, Wong LF (1995) Matrix swelling: a simple model describing extent of swelling of HPMC matrices. Int J Pharm 116(2):159–168
Nerurkar J, Jun H, Price J, Park M (2005) Controlled-release matrix tablets of ibuprofen using cellulose ethers and carrageenans: effect of formulation factors on dissolution rates. Eur J Pharm Biopharm 61(1–2):56–68
Nagarwal RC, Ridhurkar DN, Pandit J (2010) In vitro release kinetics and bioavailability of gastroretentive cinnarizine hydrochloride tablet. AAPS PharmSciTech 11(1):294–303
Patel A, Modasiya M, Shah D, Patel V (2009) Development and in vivo floating behavior of verapamil HCl intragastric floating tablets. AAPS PharmSciTech 10(1):310–315
Ebube NK, Jones AB (2004) Sustained release of acetaminophen from a heterogeneous mixture of two hydrophilic non-ionic cellulose ether polymers. Int J Pharm 272(1–2):19–27
Shanmugam S, Chakrahari R, Sundaramoorthy K, Ayyappan T, Vetrichelvan T (2011) Formulation and evaluation of sustained release matrix tablets of Losartan potassium. Int J PharmTech Res 3(1):526–534
Juarez-Soberanez D, Villafuerte-Robles LG (2011) 39/01 as excipient for gastroretentive metronidazole sustained delivery. Int J Pharm Pharm Sci 3(2):86–91
Merchant HA, Shoaib HM, Tazeen J, Yousuf RI (2006) Once-daily tablet formulation and in vitro release evaluation of cefpodoxime using hydroxypropyl methylcellulose: a technical note. AAPS PharmSciTech 7(3):E178–E183
Sankalia JM, Sankalia MG, Mashru RC (2008) Drug release and swelling kinetics of directly compressed glipizide sustained-release matrices: establishment of level A IVIVC. J Control Release 129(1):49–58
LcS Koester, Ortega GG, Mayorga P, Bassani VL (2004) Mathematical evaluation of in vitro release profiles of hydroxypropylmethylcellulose matrix tablets containing carbamazepine associated to β-cyclodextrin. Eur J Pharm Biopharm 58(1):177–179
Papadopoulou V, Kosmidis K, Vlachou M, Macheras P (2006) On the use of the Weibull function for the discernment of drug release mechanisms. Int J Pharm 309(1–2):44–50
Peppas N (1985) Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv 60(4):110–1
Rao GK, Mandapalli PK, Manthri R, Reddy VP (2013) Development and in vivo evaluation of gastroretentive delivery systems for cefuroxime axetil. Saudi Pharm J 21(1):53–59
Tillotson JK (2004) Development and evaluation of extended-release bumetanide tablets. University of Cincinnati
Bajaj S, Singla D, Sakhuja N (2012) Stability testing of pharmaceutical products. J Appl Pharm Sci 2:129–138
Júlio TA, Zâmara IF, Garcia JS, Trevisan MG (2013) Compatibility and stability of valsartan in a solid pharmaceutical formulation. Braz J Pharm Sci 49(4):645–651
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghayas, S., Shoaib, M.H., Qazi, F. et al. Influence of different viscosity grade cellulose-based polymers on the development of valsartan controlled release tablets. Polym. Bull. 77, 1281–1306 (2020). https://doi.org/10.1007/s00289-019-02802-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02802-2