Abstract
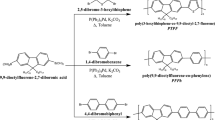
Pendant carboxyl groups were introduced into donor–acceptor polyfluorene carrying benzothiadiazole as an acceptor moiety. The emission color from its solution was different depending on the solvent. While the methanol solution of the polymer exhibited blue emission, yellow emission was observed from its tetrahydrofuran solution. Influence of solvent polarity, solution concentration, temperature, and addition of acid on photoluminescence properties indicated that the observed change in emission color was due to polymer aggregation coming from inter and/or intramolecular interaction between pendant carboxyl groups. Polyfluorene carrying benzothiadiazole moiety with pendant amino groups also exhibited emission color change and was useful as a fluorimetric sensor for polyacid.
















Similar content being viewed by others
References
Arias AC, MacKenzie JD, McCulloch I, Rivnay J, Salleo A (2010) Materials and applications for large area electronics: solution-based approaches. Chem Rev 110:3–24
Coropceanu V, Cornil J, da Silva Filho DA, Olivier Y, Silbey R, Brédas J-L (2007) Charge transport in organic semiconductors. Chem Rev 107:926–952
Zaumseil J, Sirringhaus H (2007) Electron and ambipolar transport in organic field-effect transistors. Chem Rev 107:1296–1323
Cheng Y-J, Yang S-H, Hsu C-S (2009) Synthesis of conjugated polymers for organic solar cell applications. Chem Rev 109:5868–5923
Günes S, Neugebauer H, Sariciftci NS (2007) Conjugated polymer-based organic solar cells. Chem Rev 107:1324–1338
Clarke TM, Durrant JR (2010) Charge photogeneration in organic solar cells. Chem Rev 110:6736–6767
Lee JF, Hsu SLC, Lee PI, Chuang HY, Yang ML, Chen JS, Chou WY (2010) A new intramolecular donor–acceptor polyfluorene copolymer for bulk heterojunction solar cells. Sol Energy Mater Sol Cells 94:1166–1172
Smith KA, Lin Y-H, Mok JW, Yager KG, Strzalka J, Nie W, Mohite AD, Verduzco R (2015) Molecular origin of photovoltaic performance in donor-block-acceptor all-conjugated block copolymers. Macromolecules 48:8346–8353
Cimrová V, Morávková Z, Pokorná V, Výprachický D (2017) Investigation of donor–acceptor copolymer films and their blends with fullerene in the active layers of bulk heterojunction solar cells by Raman microspectroscopy. Org Electron 47:194–199
Crossley DL, Urbano L, Neumann R, Brourke S, Jones J, Dailey LA, Green M, Humphries MJ, King SM, Turner ML (2017) Post-polymerization C–H borylation of donor–acceptor materials gives highly efficient solid state near-infrared emitters for near-IR-OLEDs and effective biological imaging. ACS Appl Mater Interfaces 9:28243–28249
Altınok E, Smith ZC, Thomas SW (2015) Two-dimensional, acene-containing conjugated polymers that show ratiometric fluorescent response to singlet oxygen. Macromolecules 48:6825–6831
Nakamura M, Yamabuki K, Oishi T, Onimura K (2013) Synthesis and fluorescent properties of conjugated copolymers containing maleimide and fluorene units at the main chain. J Polym Sci Part A Polym Chem 51:4945–4956
Yang J, Jiang C, Zhang Y, Yang R, Yang W, Hou Q, Cao Y (2004) High-efficiency saturated red emitting polymers derived from fluorene and naphthoselenadiazole. Macromolecules 37:1211–1218
Wang F, Bazan GC (2006) Aggregation-mediated optical properties of pH-responsive anionic conjugated polyelectrolytes. J Am Chem Soc 128:15786–15792
Yang R, Tian R, Yan J, Zhang Y, Yang J, Hou Q, Yang W, Zhang C, Cao Y (2005) Deep-red electroluminescent polymers: synthesis and characterization of new low-band-gap conjugated copolymers for light-emitting diodes and photovoltaic devices. Macromolecules 38:244–253
Guo Z-S, Pei J, Zhou Z-L, Zhao L, Gibson G, Lam S, Brug J (2009) Amine groups-functionalized alcohol-soluble polyfluorene derivatives: synthesis, photophysical properties, and self-assembly behaviors. Polymer 50:4794–4800
Brookins RN, Schanze KS, Reynolds JR (2007) Base-free Suzuki polymerization for the synthesis of polyfluorenes functionalized with carboxylic acids. Macromolecules 40:3524–3526
Hong JW, Hemme WL, Keller GE, Rinke MT, Bazan GC (2006) Conjugated-polymer/DNA interpolyelectrolyte complexes for accurate DNA concentration determination. Adv Mater 18:878–882
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ando, D., Ijichi, J., Uno, T. et al. Preparation of donor–acceptor polyfluorenes with pendant carboxyl or amine functionalities and their photoluminescence properties. Polym. Bull. 76, 6137–6151 (2019). https://doi.org/10.1007/s00289-019-02701-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02701-6