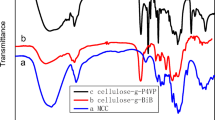
Abstract
Graft copolymers of sodium salt of carboxymethyl cellulose and N-vinylimidazole with different contents of the latter are synthesized by radical polymerization. Polymerization kinetics for all samples is researched by copolymer composition determination by UV spectroscopy, and it was found that it lasted 180 min. Percent of grafting calculated from FTIR data is in the range 31–59%, which correlated with GPC and static light scattering data. Aqueous solutions of the synthesized copolymers are characterized by dynamic light scattering (DLS), transmission electron microscopy, and zeta potential measurement. It was established that macromolecular particles of all synthesized copolymers possess non-spherical shape and negative electrokinetic potential value. The hydrodynamic radii of the polymer coils are 120–152 nm. It was found by DLS that macromolecular coils of all copolymers are stable in 0.15 M NaCl aqueous solution and in the physiological pH range which allows to apply them as vectors for targeted drug delivery. Interaction between paclitaxel (Ptx) and Na-CMC-g-PVI copolymer was studied by UV spectroscopy, FTIR, and TEM. Copolymer and Ptx complex formation proceeds by interaction of imidazole cycles and methylene backbone of Na-CMC-g-PVI copolymer and C=O groups and aromatic rings of Ptx. In vitro release kinetics was also researched in acidic and neutral media at 38 °C. Particularly, full release of paclitaxel is reached after 144 h. Release process of Ptx from copolymer particles is described by Korsmeyer–Peppas kinetic model and limited by molecular diffusion.










Similar content being viewed by others
Abbreviations
- ζ-potential:
-
Electrokinetic potential
- η :
-
Shear viscosity
- θ :
-
Scattering angle
- λ :
-
Wavelength
- ν :
-
Wavenumber
- τ :
-
Time
- τ fast :
-
Fast relaxation time
- τ slow :
-
Slow relaxation time
- A :
-
Absorbance
- A 2 :
-
Virial coefficient
- A fast :
-
Portion of scattered light relating in fast mode
- A slow :
-
Portion of scattered light relating in slow mode
- AGU:
-
Anhydrous glucose unit
- C :
-
Concentration
- C Ptx,0 :
-
Concentration of paclitaxel at the start of loading
- C Ptx, τ :
-
Concentration of paclitaxel at the τ moment
- C Na-CMC- g-PVI :
-
Concentration of synthesized copolymer
- CMC:
-
Carboxymethyl cellulose
- EE:
-
Encapsulation efficiency
- FG:
-
Frequency of grafting
- K :
-
Solution optical constant
- k 0 :
-
Zero-order release rate constant
- LE:
-
Loading efficiency
- m PVI g :
-
Mass of grafted PVI calculated from FTIR data
- m PVI t :
-
Theoretical mass of PVI
- n :
-
Diffusional exponent
- Na-CMC:
-
Sodium salt of carboxymethyl cellulose
- Na-CMC-g-PVI:
-
Graft copolymer of CMC and N-vinylimidazole
- PG:
-
Percent of grafting
- Ptx:
-
Paclitaxel
- PVI:
-
Poly-N-vinylimidazole
- q :
-
Wave vector modulus
- q 0 :
-
Initial level of the drug in the media
- q τ :
-
Level of the released drug in time τ
- q ∞ :
-
Equilibrium level of the released drug
- R θ :
-
Scattering coefficient at the θ scattering angle
- R h :
-
Hydrodynamic radius
- R fasth :
-
Hydrodynamic radius in fast mode
- R slowh :
-
Hydrodynamic radius in slow mode
- R g :
-
Radius of gyration
- R fastg :
-
Radius of gyration in fast mode
- R slowg :
-
Radius of gyration in slow mode
- T :
-
Absolute temperature
- VI:
-
N-Vinylimidazole
References
Dalmoro A, Barba A, Lamberti M, Mazzeo M (2014) Random l-lactide/ε-caprolactone copolymers as drug delivery materials. J Mater Sci 49:5986–5996. https://doi.org/10.1007/s10853-014-8317-x
Chernikova E, Sivtsov E (2017) Reversible addition-fragmentation chain-transfer polymerization: fundamentals and use in practice. Polym Sci Ser B 59:117–146. https://doi.org/10.1134/S1560090417020038
Barba A, Dalmoro A, Lamberti G (2014) Biocompatible nano-micro-particles by solvent evaporation from multiple emulsions technique. J Mater Sci 49:5160–5170. https://doi.org/10.1007/s10853-014-8224-1
Kirsh YE (1998) Water soluble poly-N-vinylamides: synthesis and physicochemical properties. Wiley, Chichester
Oh J, Drumright R, Siegwart D, Matyjaszewski K (2008) The development of microgels/nanogels for drug delivery applications. Prog Polym Sci 33:448–477. https://doi.org/10.1016/j.progpolymsci.2008.01.002
Kabanov AV, Vinogradov SV (2009) Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed 48:5418–5429. https://doi.org/10.1002/anie.200900441
Wang Y, Nie J, Chang B et al (2013) Poly(N-vinylcaprolactam)-based biodegradable multiresponsive microgels for drug delivery. Biomacromol 14:3034–3046. https://doi.org/10.1021/bm401131w
Edgar K, Buchanan C, Debenham JS et al (2001) Advances in cellulose ester performance and application. Prog Polym Sci 26:1605–1688. https://doi.org/10.1016/S0079-6700(01)00027-2
Barbucci R, Leone G, Vecchiullo A (2004) Novel carboxymethylcellulose-based microporous hydrogels suitable for drug delivery. J Biomat Sci Polym Ed 15:607–619. https://doi.org/10.1163/156856204323046870
Rasoulzadeh M, Namaz H (2017) Carboxymethyl cellulose/graphene oxide bio-nanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr Polym 168:320–326. https://doi.org/10.1016/j.carbpol.2017.03.014
Abdulkhani A, Sousefi M, Ashori A (2016) Preparation and characterization of sodium carboxymethyl cellulose/silk fibroin/graphene oxide nanocomposite films. Polymer Test 52:218–224. https://doi.org/10.1016/j.polymertesting.2016.03.020
Shatalov GV, Lavlinskaya MS, Pakhomova OA et al (2016) Copolymers of N-vinylcaprolactam with 1-vinyl- and 1-methacryloyl-3,5-dimethylpyrazole as sorbents of essential α-amino acids in liquid- and solid-phase extraction. Russ J Appl Chem 89:140–146. https://doi.org/10.1134/S1070427216010225
Kuznetsov VA, Lavlinskaya MS, Ostankova IV et al (2018) Synthesis of N-vinylformamide and 1-vinyl-(1-methacryloyl)-3,5-dimethylpyrazole copolymers and their extraction ability in relation to histidine in water-salt media. Polym Bull 75:1237–1251. https://doi.org/10.1007/s00289-017-2091-2
Atanase L, Desbrieres J, Riess G (2017) Micellization of synthetic and polysaccharides-based graft copolymers in aqueous media. Prog Polym Scie 73:32–60. https://doi.org/10.1016/j.progpolymsci.2017.06.001
Bhattacharyya S, Maldas D (1984) Graft copolymerization onto cellulosics. Prog Polym Sci 10:171–270. https://doi.org/10.1016/0079-6700(84)90002-9
McDowall D, Gupta B, Stannett V (1984) Grafting of vinyl monomers to cellulose by ceric ion initiation. Prog Polym Sci 10:1–50. https://doi.org/10.1016/0079-6700(84)90005-4
Bhattacharya A, Misra B (2004) Grafting: a versatile means to modify polymers: techniques, factors and applications. Prog Polym Sci 29:767–814. https://doi.org/10.1016/j.progpolymsci.2004.05.002
Genç F, Uzun C, Güven O (2016) Quaternized poly(1-vinylimidazole) hydrogel for anion adsorption. Polym Bull 73:179–190. https://doi.org/10.1007/s00289-015-1479-0
Molina M, Gomez-Anton M, Rivas B et al (2001) Removal of Hg(II) from acid aqueous solutions by poly(N-vinylimidazole) hydrogel. J Appl Polym Sci 79:1467–1475
Yin Y, Dong Z, Luo Q et al (2012) Biomimetic catalysts designed on macromolecular scaffolds. Prog Polym Sci 11:1476–1509. https://doi.org/10.1016/j.progpolymsci.2012.04.001
Beletskaya I, Tarasenko E, Khokhlov A et al (2007) Poly(N-vinylimidazole) as efficient and recyclable catalyst for the addition of thiols to michael acceptors in aqueous medium. Russ J Org Chem 43:1733–1736. https://doi.org/10.1134/S1070428007110279
Barabanova A, Blagodatskikh I, Vyshivannaya O et al (2015) Catalytic properties of diblock copolymers of N-vinylcaprolactam and N-vinylimidazole. Dokl Chem 465:253–256. https://doi.org/10.1134/S0012500815110014
Bellamy LJ (1958) The infra-red spectra of complex molecules. Methuen & Co., Ltd/Wiley, London/New York
Gupta K, Sahoo S, Khandekar K (2002) Graft copolymerization of ethyl acrylate onto cellulose using ceric ammonium nitrate as initiator in aqueous medium. Biomacromol 3:1087–1094. https://doi.org/10.1021/bm020060s
Kuznetsov VA, Kushchev PO, Blagodatskikh IV et al (2016) Aqueous dispersions of cross-linked poly-N-vinylcaprolactam stabilized with hydrophobically modified polyacrylamide: synthesis, colloidal stability, and thermosensitive properties. Colloid Polym Sci 294:889–899. https://doi.org/10.1007/s00396-016-3843-5
Jakubiak-Marcinkowska A, Legan M, Jezierska J (2013) Molecularly imprinted polymeric Cu(II) catalysts with modified active centres mimicking oxidation enzyme. J Polym Res 20:317–328. https://doi.org/10.1007/s10965-013-0317-z
Özbas Z, Ökahraman B, Öztürk AB (2018) Controlled release profile of 5-fluorouracil loaded P(AAM-co-NVP-co-DEAEMA) microgel prepared via free radical precipitation polymerization. Polym Bull. https://doi.org/10.1007/s00289-017-2202-0
Korsmeyer RW, Gurny R, Doelker EM, Buri P, Peppas NA (1983) Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 15:25–35. https://doi.org/10.1016/0378-5173(83)90064-9
Xu J, Xu B, Shou D, Xia X, Hu Y (2015) Preparation and evaluation of vancomycin-loaded N-trimethyl chitosan nanoparticles. Polymers 7:1850–1870. https://doi.org/10.3390/polym7091488
Llabot JM, Manzo RH, Allemandi DA (2004) Drug release from carbomer: carbomer sodium salt matrices with potential use as mucoadhesive drug delivery system. Int J Pharm 276:59–66. https://doi.org/10.1016/j.ijpharm.2004.02.006
Acknowledgements
FTIR, TEM, UV, zeta potential data were obtained with the use of equipment of Centre of Collective Usage of Scientific Equipment of Voronezh State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kuznetsov, V.A., Sorokin, A.V., Lavlinskaya, M.S. et al. Graft copolymers of carboxymethyl cellulose with N-vinylimidazole: synthesis and application for drug delivery. Polym. Bull. 76, 4929–4949 (2019). https://doi.org/10.1007/s00289-018-2635-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2635-0