Abstract
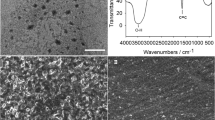
Here, a molecularly imprinted electrochemical quartz crystal microbalance (MIP-EQCM) sensor for aspartame is developed by grafting the aspartame-imprinted polymeric matrix of chitosan on gold-coated quartz crystal electrode. Chitosan nanoparticles being biocompatible, biodegradable and also having large surface area provide a better platform by forming a well-dispersed composite suspension with graphene. Additionally graphene facilitates direct electron transfer to electrode surface for electrochemical study because of having enhanced electrical conductivity. This EQCM-MIP sensor was characterized by atomic force microscopy, contact angle measurements, cyclic voltammetry and differential pulse voltammetry (DPV). The obtained MIP showed high affinity to aspartame. A reliable method for analysis of aspartame in real and commercial samples was achieved by coupling EQCM-MIP with DPV. Linear relationship with R2 = 0.9749 (EQCM) and R2 = 0.9760 (DPV) on binding of aspartame at various concentrations was observed. Detection limit of 0.45 µg mL−1 (EQCM) and 0.07 µg mL−1 (DPV) of the fabricated sensor shows that high sensitivity and high selectivity among various structural analogues of aspartame were also achieved. The improved detection limit is promising for determination of trace amount of aspartame. This demonstrates good memory capacity of this EQCM sensor. High recovery percentage and applicability of EQCM-MIP sensor in real matrices and commercial samples offers good potential for various applications.











Similar content being viewed by others
References
Xu SF, Li JH, Song XL, Liu JS, Lu HZ, Chen LX (2013) Photonic and magnetic dual responsive molecularly imprinted polymers: preparation, recognition, characteristics and properties as a novel sorbent for caffeine in complicated samples. Anal Methods 5:124–133
Zhou CH, Wang TT, Liu JQ, Guo C, Peng Y, Bai JL, Liu M, Dong JW, Gao N, Ning BA, Gao ZX (2012) Molecularly imprinted photonic polymer as an optical sensor to detect chloramphenicol. Analyst 137:4469–4474
Wang YT, Zhang ZQ, Jain V, Yi JJ, Mueller S, Sokolov J, Liu ZX, Levon K, Rigas B, Rafailovich MH (2010) Potentiometric sensor based on surface molecular imprinting: detection of cancer biomarkers and viruses. Sens Actuat B Chem 146:381–387
Srivastava J, Singh M (2016) A biopolymeric nano-receptor for sensitive and selective recognition of albendazole. Anal Methods 8:1026–1033
Singh M, Kumar A, Tarannum N (2013) Water compatible aspartame-imprinted polymer grafted on silica surface for selective recognition in aqueous solution. Anal Bioanal Chem 405:4245–4252
Tarannum N, Singh M (2012) Water-compatible surface imprinting of baclofen on silica surface for selective recognition and detection in aqueous solution. Anal Methods 4:3019–3026
Slonczewski JC, Weiss PR (1958) Band structure of graphite. Phys Rev 109:272–279
Kim H, Abdala AA, Macosko CW (2010) Graphene/polymer nanocomposite. Macromolecules 43:6515–6530
Han D, Yan L, Chen W, Li W (2011) Preparation of chitosan/graphene oxide composite film with enhanced mechanical strength in the wet state. Carbohydr Polym 83:653–658
Artiles MS, Rout CS, Fisher TS (2011) Graphene based hybrid materials and devices for biosensing. Adv Drug Deliv Rev 63:1352–1360
Singh V, Joung D, Zhai L, Das S, Khondaker SI, Seal S (2011) Graphene based materials: past, present and future. Prog Mater Sci 56:1178–1271
Luo J, Jiang S, Liu X (2014) Electrochemical sensor for bovine hemoglobin based on a novel graphene molecular-imprinted polymers composite as recognition element. Sens Actuat B Chem 203:782–789
Kang X, Wang J, Wu H, Aksay IA, Liu J, Lin Y (2009) Glucose oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens Bioelectron 25:901–905
Pierini GD, Llamas NE, Fragoso WD, Lemos SG, Di Nezio MS, Centurion ME (2013) Simultaneous determination of acesulfame-K and aspartame using linear sweep voltammetry and multivariate calibration. Microchem J 106:347–350
Drewnowski A, Massien C, Louis-Sylvestre J, Fricker J, Chapelot D, Apfelbaum M (1994) Comparing the effects of aspartame and sucrose on motivational ratings, taste preferences and energy intakes in humans. Am J Clin Nutr 59:338–345
Lean MEJ, Hankey CR (2004) Aspartame and its effects on health. BMJ 329:755–756
Blackburn GL, Kanders BS, Lavin PT, Keller SD, Whatley J (1997) The effect of aspartame as part of a multidisciplinary weight control on short-and long-term control of body weight. Am J Clin Nutr 65:409–418
Ranney RE, Oppermann JA (1979) A review of metabolism of aspartyl moiety of aspartame in experimental animals and man. J Environ Pathol Toxicol 2:979–985
Ranney RE, Oppermann JA, Muldoon E, McMahon FG (1976) The comparative metabolism of aspartame in experimental animals and man. J Toxicol Environ Health 2:441–451
Oppermann JA (1984) Aspartame physiology and biochemistry. Marcel Dekker Inc., New York, pp 141–159
Marinovich M, Galli CL, Bosetti C, Gallus S, La Vecchia C (2013) Aspartame, low calorie sweeteners and disease: regulatory safety and epidemiological issues. Food Chem Toxicol 60:109–115
Soffritti M, Belpoggi F, Esposti DD, Lambertini L, Tibaldi E, Rigano A (2006) First experimental demonstration of multipotential carcinogenic effects of aspartame administrated in the feed to Sprague-Dawley rats. Environ Health Perspect 114:379–385
Soffritti M, Belpoggi F, Tibaldi E, Esposti DD, Lauriola M (2007) Life-span exposure to low doses of aspartame beginning during prenatal life increases cancer effects in rats. Environ Health Perspect 115:1293–1297
Humphries P, Pretorius E, Naude H (2007) Direct and cellular effects of aspartame on brain. Eur J Clin Nutr 62:451–462
Coulombe RA Jr, Sharma RP (1986) Neurochemical alterations induced by the artificial sweetener aspartame (NutraSweet). Toxicol Appl Pharmacol 83:79–85
Rashidian M, Fattahi A (2009) Comparison of thermochemistry of aspartame (artificial sweetener) and glucose. Carbohydr Res 344:127–133
Newman LC, Lipton RB (2001) Migraine MLT-down: an unusual presentation of migraine in patients with aspartame-triggered headaches. Headache 41:899–901
Lipton RB, Newman LC, Cohen JS, Solomon S (1989) Aspartame as a dietary trigger of headache. Headache 29:90–92
Tsakiris S, Giannoulia-Karantana A, Simintzi I, Schulpis KH (2006) The effects of aspartame metabolites on human erythrocyte membrane acetylcholinesterase activity. Pharmacol Res 53:1–5
Dinckaya E, Cagin M, Telefoncu A (1994) Enzymatic method for the spectrophotometric determination of aspartame. Food Chem 50:95–97
de Ndbrega JA, Fatibello-Filho O, da Vieira IC (1994) Flow injection spectrophotometry determination of aspartame in dietary products. Analyst 119:2101–2104
Furda I, Malizia PD, Kolor MG, Vemieri PJ (1975) Decomposition products of l-aspartyl l-phenylalanine methyl ester and their identification by gas–liquid chromatography. J Agric Food Chem 23:340–343
Tsang W, Clarke MA, Parrish FW (1985) Determination of aspartame and its breakdown products in soft drinks by reverse-phase chromatography with UV detection. J Agric Food Chem 33:734–738
Garcia Sanchez F, Aguilar Gallardo A (1992) Liquid chromatographic and spectrofluorimetric determination of aspartame and glutamate in foodstuffs following flourescamine flourigenic labelling. Anal Chim Acta 270:45–53
Kim SK, Jung MY, Kim SY (1997) Photodecomposition of aspartame in aqueous solutions. Food Chem 59:273–278
Zhua Y, Guo Y, Ye M, James FS (2005) Separation and simultaneous determination of four artificial sweeteners in food and beverages by ion chromatography. J Chromatogr A 1085:143–146
Zygler A, Wasik A, Kot-Wasik A, Namiesnik J (2011) Determination of nine high-intensity sweeteners in various foods by high-performance liquid chromatography with mass specrometric detection. Anal Bioanal Chem 400:2159–2172
Kumari A, Choudhary S, Arora S, Sharma V (2016) Stability of aspartame and neotame in pasteurized and in-bottle sterilized flavoured milk. Food Chem 196:533–538
Tosa N, Moldovan Z, Bratu I (2011) Simultaneous determination of some artificial sweeteners in ternary formulations by FT-IR and EI-MS. AIP Conf Proc 1425:98–101
Herzog G, Kam V, Berduque A, Arrigan DWM (2008) Detection of food additives by voltammetry at liquid–liquid interface. J Agric Food Chem 56:4304–4310
Antigo Medeiros R, Evaristo de Carvalho A, Rocha-Filho RC, Fatibello-Filho O (2008) Simultaneous square-wave voltammetric determination of aspartame and cyclamate using a boron-doped diamond electrode. Talanta 76:685–689
Cantarelli MA, Pellerano RG, Marchevsky EJ, Camina JM (2009) Simultaneous determination of aspartame and acesulfame-K by molecular absorption spectrophotometry using multivariate calibration and validation by high performance liquid chromatography. Food Chem 115:1128–1132
Pesek JJ, Matyska MT (1997) Determination of aspartame by high-performance electrophoresis. J Chromatogr A 781:423–428
Stojkovic M, Mai TD, Hauser PC (2013) Determination of artificial sweeteners by capillary electrophoresis with contactless conductivity detection optimized by hydrodynamic pumping. Anal Chim Acta 787:254–259
Bergamo AB, Fracassi da Silva JA, Pereira de Jesus D (2011) Simultaneous determination of aspartame, cyclamate, sachharin and acesulfame-K in softdrinks and table top sweetener formulations by capillary electrophoresis with capacitively coupled contactless conductivity detection. Food Chem 124:1714–1717
Becker B et al (2011) A survey of 2006–2009 quartz crystal microbalance biosensor literature. J Mol Recognit 24:754–787
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814
Umrao S, Gupta TK, Kumar S, Singh VK, Sultania MK, Jung JH, Oh IK, Srivastava A (2015) Microwave-assisted synthesis of boron and nitrogen co-doped reduced graphene oxide for the protection of electromagnetic radiation in Ku band. Mater Interfaces 7:19831–19842
Pan Y, Li YJ, Zhao HY, Zheng JM, Xu H, Wei G, Hao JS, Cui FD (2002) Bioadhesive polysachharide in protein delivery system: chitosan nanoparticles improves the intestinal absorption of insulin in vivo. Int J Pharm 249:139–147
Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ (1997) Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci 63:125–132
Janes KA, Fresneau MP, Marazuela A, Fabra A, Alonso MJ (2001) Chitosan nanoparticles as delivery system for doxorubicin. J Control Release 73:255–267
Bodmeier R, Chen HG, Paeratakul O (1989) A novel approach to the oral delivery of micro or nanoparticle. Pharm Res 6:413–417
Schniepp HC, Li JL, Mcallister MJ, Sai H, Herrera-Alonso M, Adamson DH, Prud’homme RK, Car R, Saville DA, Aksay IA (2006) Functionalized single graphene sheets derived from splitting graphene oxide. J Phys Chem B 110:8535–8539
Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS (2006) Graphene-based composite materials. Nature 442:282–286
Lian W, Liu S, Yu J, Xing X, Li J, Cui M, Huang J (2012) Electrochemical sensor based on gold nanoparticles fabricated molecularly imprinted film at chitosan–platinum nanoparticle/graphene–gold nanoparticle double nanocomposite modified electrode for detection of erythromycin. Biosens Bioelectron 38:163–169
Spector MS, Naranjo E, Chiruvolu S, Zasadzinski JA (1994) Conformations of a tethered membrane: crumpling in graphitic oxide? Phys Rev Lett 73:2867–2870
Cakir S, Coskun E, Bicer E, Cakir O (2003) Electrochemical study of the complexes of aspartame with Cu(II), Ni(II) and Zn(II) ions in the aqueous medium. Carbohydr Res 338:1217–1222
Deroco PB, Medeiros RA, Rocha-Filho RC, Fatibello-Filho O (2015) Simultaneous voltammetric determination of aspartame and acesulfame-K in food products using an anodically pretreated boron-doped diamond electrode. Anal Methods 7:2135–2140
Rowe RC, Sheskey PJ, Weller PJ (2003) Handbook of pharmaceutical excipients, 4th edn. American Pharmaceutical Association, Washington, pp 37–39
Bell LN, Labuza TP (1991) Aspartame degradation kinetics as affected by pH in intermediate and low moisture food systems. J Food Sci 56:17–20
Ozol T (1986) Stability of aspartame in artificial syrup. Acta Pharm True 28:125–128
Dougherty DA (1996) Cation–π interactions in chemistry and biology: a new view of Benzene, Phe, Tyr and Trp. Science 271:163–168
Gloria MBA (2003) Encyclopedia of food science and nutrition, 2nd edn. Academic Press Publisher, pp 332–338. ISBN: 9780080917917
Radulescu MC, Bucur B, Bucur MP, Radu GL (2014) Bienzymatic biosensor for rapid detection of aspartame by flow injection analysis. Sensors 14:1028–1038
Tiua BDB, Pernitesb RB, Tiua SB, Advinculaa RC (2016) Detection of aspartame via microsphere patterned and molecularly imprinted polymer arrays. Colloids Surf A Physicochem Eng Aspects 495:149–158
Acknowledgements
Authors acknowledge Department of Chemistry, Banaras Hindu University for AFM analysis and the financial support by the DST-SERB, New Delhi (Grant No. EMR/2016/005245).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Srivastava, J., Gupta, N., Kushwaha, A. et al. Highly sensitive and selective estimation of aspartame by chitosan nanoparticles–graphene nanocomposite tailored EQCM-MIP sensor. Polym. Bull. 76, 4431–4449 (2019). https://doi.org/10.1007/s00289-018-2597-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2597-2