Abstract
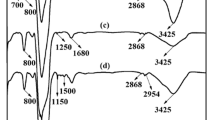
A new adsorbent was synthesized by functionalizing poly(glycidyl methacrylate) with 2,6-diaminopyridine for the selective adsorption of Au(III) from aqueous solution. The fourier transform infrared spectrometer, X-ray photoelectron spectroscopy and scanning electron microscope demonstrated that 2,6-diaminopyridine was successfully grafted onto poly(glycidyl methacrylate). The batch adsorption experiments were conducted to evaluate the adsorption abilities of the adsorbent. The results show that the adsorbent exhibits high adsorption capacity (459.29 mg/g) for Au(III) at pH 4. The adsorption process of Au(III) are well described by pseudo-second-order kinetic model. The adsorption isotherm is well-obeyed Langmuir and D–R models. The calculated thermodynamic parameters of the adsorption process are: ΔG0 = − 24.2 kJ/mol; ΔH0 = 37.0 kJ/mol; ΔS0 = 205.4 J/mol K. The adsorbent is interacted with Au(III) through chelation and ion exchange between the amines groups and Au(III). Moreover, the adsorbent shows good selectivity from the interfering ions and can be reused at least five times.












Similar content being viewed by others
References
Campos PG, Grimalt JO, Berdie L, Lopez-Quintero JO, Navarrete-Reyes LE (2008) Gold reserves of central banks and governments. Org Geochem 25(8):475–488
Pourreza N, Rastegarzadeh S (2005) Selective transport of gold through liquid membrane using 5-(p-dimethylaminobenzylidene)rhodanine as carrier. Chem Anal (Warsaw) 50(4):695–703
Peng L, Guang-feng L, Da-lin C, Shao-yi C, Ning T (2009) Adsorption properties of Ag(I), Au(III), Pd(II) and Pt(IV) ions on commercial 717 anion-exchange resin. Trans Nonferrous Met Soc 19(6):1509–1513
Das N (2010) Recovery of precious metals through biosorption—a review. Hydrometallurgy 103(1):180–189
Spitzer M, Bertazzoli R (2004) Selective electrochemical recovery of gold and silver from cyanide aqueous effluents using titanium and vitreous carbon cathodes. Hydrometallurgy 74(3–4):233–242
Syed S (2012) Recovery of gold from secondary sources—a review. Hydrometallurgy 115–116(4):30–51
Skrabalak SE, Chen J, Au L, Lu X, Li X, Xia Y (2007) Gold nanocages for biomedical applications. Adv Mater 19(20):3177–3184
Ma G, Yan W, Chen J, Yan C, Shi N, Wu J, Xu G (2000) Progress in gold solvent extraction. Prog Nat Sci Mater Int 10(12):881–886
Fu L, Zhang L, Wang S, Zhang B, Peng J (2017) Selective recovery of Au(III) from aqueous solutions by nanosilica grafted with cationic polymer: kinetics and isotherm. J Taiwan Inst Chem Eng 80:342–348
Baker J, Baker RJ, Schulzke C (2011) Perfluorinated oxygen- and sulfur-containing compounds as extractants for gold(III). Gold Bull 44(2):79–83
Lu W, Lu Y, Liu F, Shang K, Wang W, Yang Y (2011) Extraction of gold(III) from hydrochloric acid solutions by CTAB/n-heptane/iso-amyl alcohol/Na2SO3 microemulsion. J Hazard Mater 186(2–3):2166–2170
Jainae K, Sanuwong K, Nuangjamnong J, Sukpirom N, Unob F (2010) Extraction and recovery of precious metal ions in wastewater by polystyrene-coated magnetic particles functionalized with 2-(3-(2-aminoethylthio)propylthio)ethanamine. Chem Eng J 160(2):586–593
Lam KF, Chi MF, Yeung KL (2007) Separation of precious metals using selective mesoporous adsorbents. Gold Bull 40(3):192–198
Chang YC, Chen DH (2006) Recovery of gold (III) ions by a chitosancoated magnetic nano-adsorbent. Gold Bull 39(3):98–102
Gomes CP, Almeida MF, Loureiro JM (2001) Gold recovery with ion exchange used resins. Sep Purif Technol 24(1–2):35–57
Al-Merey R, Hariri Z, Hilal JA (2003) Selective separation of gold from iron ore samples using ion exchange resin. Microchem J 75(3):169–177
Donia AM, Atia AA, El-Boraey HA, Mabrouk DH (2006) Adsorption of Ag(I) on glycidyl methacrylate/N,N′-methylene bis-acrylamide chelating resins with embedded iron oxide. Sep Purif Technol 48(3):281–287
Donia AM, Atia AA, Elwakeel KZ (2007) Recovery of gold(III) and silver(I) on a chemically modified chitosan with magnetic properties. Hydrometallurgy 87(3–4):197–206
Hasanzadeh R, Moghadam PN, Bahri-Laleh N, Sillanpää M (2017) Effective removal of toxic metal ions from aqueous solutions: 2-bifunctional magnetic nanocomposite base on novel reactive PGMA-MAn copolymer@Fe3O4 nanoparticles. J Colloid Interface Sci 490:727–746
Li P, Yang L, He X, Wang J, Kong P (2012) Synthesis of PGMA microspheres with amino groups for high-capacity adsorption of Cr(VI) by cerium initiated graft polymerization. Chin J Chem Eng 20(1):95–104
Liu C, Bai R (2010) Extended study of DETA-functionalized PGMA adsorbent in the selective adsorption behaviors and mechanisms for heavy metal ions of Cu Co, Ni, Zn, and Cd. J Colloid Interface Sci 350(1):282–289
Lin G, Wang S, Zhang L, Hu T, Peng J, Cheng S, Fu L, Srinivasakannan C (2018) Selective recovery of Au(III) from aqueous solutions using 2-aminothiazole functionalized corn bract as low-cost bioadsorbent. J Clean Prod 196:1007–1015
Lin G, Wang S, Zhang L, Hu T, Peng J, Cheng S, Fu L (2018) Synthesis and evaluation of thiosemicarbazide functionalized corn bract for selective and efficient adsorption of Au(III) from aqueous solutions. J Mol Liq 258:235–243
Ho YS, Mckay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34(3):735–742
Yurdakoç M, Seki Y, Karahan S, Yurdakoç K (2005) Kinetic and thermodynamic studies of boron removal by Siral 5, Siral 40, and Siral 80. J Colloid Interface Sci 286(2):440–446
Wu FC, Tseng RL (2001) Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res 35(3):613–618
Atia AA (2005) Adsorption of silver(I) and gold(III) on resins derived from bisthiourea and application to retrieval of silver ions from processed photo films. Hydrometallurgy 80(1–2):98–106
Huo HY, Su HJ, Tan TW (2009) Adsorption of Ag + by a surface molecular-imprinted biosorbent. Chem Eng J 150(1):139–144
Abdel-Rahman LH, Abu-Dief AM, Newair EF, Hamdan SK (2016) Some new nano-sized Cr(III), Fe(II), Co(II), and Ni(II) complexes incorporating 2-((E)-(pyridine-2-ylimino)methyl)napthalen-1-ol ligand: structural characterization, electrochemical, antioxidant, antimicrobial, antiviral assessment and DNA interaction. J Photochem Photobiol, B 160:18–31
Zhang L, Liu Y, Wang S, Liu B, Peng J (2015) Selective removal of cationic dyes from aqueous solutions by an activated carbon-based multicarboxyl adsorbent. RSC Adv 5(121):99618–99626
Huang J, Huang K, Liu S, Luo Q, Shi S (2008) Synthesis, characterization, and adsorption behavior of aniline modified polystyrene resin for phenol in hexane and in aqueous solution. J Colloid Interface Sci 317(2):434–441
Sun C, Qu R, Ji C, Wang C, Sun Y, Yue Z, Cheng G (2006) Preparation and adsorption properties of crosslinked polystyrene-supported low-generation diethanolamine-typed dendrimer for metal ions. Talanta 70(1):14–19
Manzoori JL, Amjadi M, Hallaj T (2009) Preconcentration of trace cadmium and manganese using 1-(2-pyridylazo)-2-naphthol-modified TiO2 nanoparticles and their determination by flame atomic absorption spectrometry. Int J Environ Anal Chem 89(8–12):749–758
Zhou L, Liu J, Liu Z (2009) Adsorption of platinum(IV) and palladium(II) from aqueous solution by thiourea-modified chitosan microspheres. J Hazard Mater 172(1):439–446
Nagireddi S, Katiyar V, Uppaluri R (2017) Pd(II) adsorption characteristics of glutaraldehyde cross-linked chitosan copolymer resin. Int J Biol Macromol 94(Pt A):72
Morcali MH, Zeytuncu B (2015) Investigation of adsorption parameters for platinum and palladium onto a modified polyacrylonitrile-based sorbent. Int J Miner Process 137:52–58
Chassary P, Vincent T, Marcano JS, Macaskie LE, Guibal E (2005) Palladium and platinum recovery from bicomponent mixtures using chitosan derivatives. Hydrometallurgy 76(1–2):131–147
Dong Z, Liu J, Yuan W, Yi Y, Zhao L (2016) Recovery of Au(III) by radiation synthesized aminomethyl pyridine functionalized adsorbents based on cellulose. Chem Eng J 283:504–513
Li H, Wang X, Cao L, Zhang X, Yang C (2015) Gold-recovery PVDF membrane functionalized with thiosemicarbazide. Chem Eng J 280:399–408
Kawakita H, Yamauchi R, Parajuli D, Ohto K, Harada H, Inoue K (2008) Recovery of gold from hydrochloric acid by means of selective coagulation with persimmon extract. Sep Sci Technol 43(9–10):2375–2385
Ngah WS, Fatinathan S (2010) Adsorption characterization of Pb(II) and Cu(II) ions onto chitosan-tripolyphosphate beads: kinetic, equilibrium and thermodynamic studies. J Environ Manage 91(4):958–969
Fujiwara K, Ramesh A, Maki T, Hasegawa H (2007) Adsorption of platinum(IV), palladium(II) and gold(III) from aqueous solutions onto l-lysine modified crosslinked chitosan resin. J Hazard Mater 146(1–2):39
Ramesh A, Hasegawa H, Sugimoto W, Maki T, Ueda K (2008) Adsorption of gold(III), platinum(IV) and palladium(II) onto glycine modified crosslinked chitosan resin. Biores Technol 99(9):3801–3809
Nguyen C, Do DD (2001) The Dubinin–Radushkevich equation and the underlying microscopic adsorption description. Carbon 39(9):1327–1336
Turanov AN, Karandashev VК, Artyushin OI, Sharova EV, Genkina GK (2017) Adsorption of palladium(II) from hydrochloric acid solutions using polymeric resins impregnated with novel N-substituted 2-(diphenylthiophosphoryl)acetamides. Sep Purif Technol 187:355–364
Pang SK, Yung KC (2014) Prerequisites for achieving gold adsorption by multiwalled carbon nanotubes in gold recovery. Chem Eng Sci 107(107):58–65
Wang X, Xu J, Li L, Li H, Yang C (2017) Thiourea grafted PVDF affinity membrane with narrow pore size distribution for Au(III) adsorption: preparation, characterization, performance investigation and modeling. Chem Eng J 314:700–713
Fan R, Xie F, Guan X, Zhang Q, Luo Z (2014) Selective adsorption and recovery of Au(III) from three kinds of acidic systems by persimmon residual based bio-sorbent: a method for gold recycling from e-wastes. Biores Technol 163(7):167–171
Sun C, Zhang G, Wang C, Qu R, Zhang Y, Gu Q (2011) A resin with high adsorption selectivity for Au(III): preparation, characterization and adsorption properties. Chem Eng J 172(2):713–720
Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (Nos. U1702252 and 51664037).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, B., Wang, S., Fu, L. et al. Selective high capacity adsorption of Au(III) from aqueous solution by poly(glycidyl methacrylate) functionalized with 2,6-diaminopyridine. Polym. Bull. 76, 4017–4033 (2019). https://doi.org/10.1007/s00289-018-2594-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2594-5