Abstract
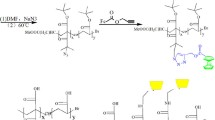
Polyurethane network 1 was prepared through curing of epoxy-based polymer bearing 1-naphthylamine units with tolylene-2,4-diisocyanate as curing agent. With ferric ions, the network was found to exhibit selective ON–OFF-type fluorescence signaling behavior even in the presence of other representative metal ions such as Na+, K+, Ag+, Cu2+, Zn2+, Co2+, Ni2+, Cd2+, Hg2+, Mg2+, Sr2+, Pb2+ and Ca2+ ions.








Similar content being viewed by others
Change history
02 September 2020
This erratum corrects the legends of Figure 3 (Inset) of the published paper. The results presented in the published paper are correct and are not affected by the change in legends of Figure 3 (inset).
References
Sie YW, Wan CF, Wu AT (2017) A multifunctional Schiff base fluorescence sensor for Hg2+, Cu2+ and Co2+ ions. RSC Adv 7:2460–2465
Neupane LN, Oh ET, Park HJ, Lee KH (2016) Selective and sensitive detection of heavy metal ions in 100% aqueous solution and cells with a fluorescence chemosensor based on peptide using aggregation-induced emission. Anal Chem 88:3333–3340
Bai L, Tou LJ, Gao Q, Bose P, Zhao Y (2016) Remarkable colorimetric sensing of heavy metal ions based on thiol-rich nanoframes. Chem Commun 52:13691–13694
Yoon S, Miller EW, He Q, Do PH, Chang CJ (2007) A bright and specific fluorescent sensor for mercury in water, cells, and tissue. Angew Chem Int Ed 46:6658–6661
Que EL, Domaille DW, Chang CJ (2008) Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev 108:1517–1549
Nolan EM, Lippard SJ (2008) Tools and tactics for the optical detection of mercuric ion. Chem Rev 108:3443–3480
Carter KP, Young AM, Palmer AE (2014) Fluorescent sensors for measuring metal ions in living systems. Chem Rev 114:4564–4601
Li M, Gou H, Ogaidi IA, Wu N (2013) Nanostructured sensors for detection of heavy metals: a review. ACS Sustain Chem Eng 1:713–723
Leitch HA, Fibach E, Rachmilewitz E (2017) Toxicity of iron overload and iron overload reduction in the setting of hematopoietic stem cell transplantation for hematologic malignancies. Crit Rev Oncol Hematol 113:156–170 (references therein)
Huang J, Xu Y, Qian X (2014) Rhodamine-based fluorescent off/on sensor for Fe3+ in aqueous solution and in living cells: 8-aminoquinoline receptor and 2:1 binding. Dalton Trans 43:5983–5989
Huang L, Hou F, Cheng J, Xi P, Chen F, Bai D, Zeng Z (2012) Selective off–on fluorescent chemosensor for detection of Fe3+ ions in aqueous media. Org Biomol Chem 10:9634–9638 (references therein)
Galaris D, Skiada V, Barbouti A (2008) Redox signaling and cancer: the role of “labile” iron. Cancer Lett 266:21–29
Ong WY, Farooqui AA (2005) Iron, neuroinflammation, and Alzheimer’s disease. J Alzheimers Dis 8:183–200
Hirayama T, Nagasawa H (2017) Chemical tools for detecting Fe ions. J Clin Biochem Nutr 60:39–48 (references therein)
Cardona MA, Mallia CJ, Baisch U, Magri DC (2016) Water-soluble amino(ethanesulfonate) and [bis(ethanesulfonate)] anthracenes as fluorescent photoinduced electron transfer (PET) pH indicators and Fe3+ chemosensors. RSC Adv 6:3783–3791
Sahoo SK, Sharma D, Bera RK, Crisponic G, Callan JF (2012) Iron(III) selective molecular and supramolecular fluorescent probes. Chem Soc Rev 41:7195–7227 (references therein)
Huang L, Hou F, Cheng J, Xi P, Chen F, Bai D, Zeng Z (2012) Selective off–on fluorescent chemosensor for detection of Fe3+ ions in aqueous media. Org Biomol Chem 10:9634–9638
Wang R, Yu RF, Liu P, Chen L (2012) A turn-on fluorescent probe based on hydroxylamineoxidation for detecting ferric ion selectively in living cells. Chem Commun 48:5310–5312
Yao JN, Dou W, Liu WS (2009) A new coumarin-based chemosensor for Fe3+ in water. Inorg Chem Commun 12:116–118
Jung HJ, Singh N, Jang DO (2008) Highly Fe3+ selective ratiometric fluorescent probe based on imine-linked benzimidazole. Tetrahedron Lett 49:2960–2964
Mao J, Wang LN, Dou W, Tang XL, Yan Y, Liu WS (2007) Tuning the selectivity of two chemosensors to Fe(III) and Cr(III). Org Lett 9:4567–4570
Xiang Y, Tong A (2006) A new rhodamine-based chemosensor exhibiting selective FeIII-amplified fluorescence. Org Lett 8:1549–1552
Bricks JL, Kovalchuk A, Trieflinger C, Nofz M, Büschel M, Tolmachev AI, Daub J, Rurack K (2005) On the development of sensor molecules that display Fe(III)-amplified fluorescence. J Am Chem Soc 127:13522–13529
Hua J, Wang TG (2005) A highly selective and sensitive fluorescent chemosensor for Fe3+ in physiological aqueous solution. Chem Lett 34:98–99
Anthony SP (2012) Polymorph-dependent solid-state fluorescence and selective metal-ion-sensor properties of 2-(2-Hydroxyphenyl)-4(3H)-quinazolinone. Chem Asian J 7:374–379
Lin WY, Long LL, Yuan L, Cao ZM, Feng JB (2009) A novel ratiometric fluorescent Fe3+ sensor based on a phenanthroimidazole chromophore. Anal Chim Acta 634:262–266
Ji X, Yao Y, Li J, Yan X, Huang F (2013) A supramolecular cross-linked conjugated polymer network for multiple fluorescent sensing. J Am Chem Soc 135:74–77
Liou GS, Lin SM, Yen HJ (2008) Synthesis and photoluminescence properties of novel polyarylates bearing pendent naphthylamine chromophores. Eur Polym J 44:2608–2618
Ghosh S, Dey CK, Manna R (2010) Epoxy-based polymer bearing 1-naphthylamine units: highly selective fluorescent chemosensor for ferric ion. Tetrahedron Lett 51:3177–3180
Ghosh S, Manna R (2011) Epoxy-based oligomer containing dithia-aza-based naphthylazobenzene pendant: a chemosensor for Hg2+ and Cu2+ ions. Supramol Chem 23:558–562
Ghosh S, Dey CK (2014) Epoxy based polymer bearing activated 3-arylazopyridine unit as a chromogenic probe of Hg2 + Ion. J Macromol Sci Pure Appl Chem Part-A 51:217–222
Ghosh S, Manna R (2014) Epoxy-based polymer bearing triphenylamine units: a highly selective fluorescent chemosensor for Hg2+ ions. RSC Adv 4:5798–5802
Lohani CR, Lee KH (2010) The effect of absorbance of Fe3+ on the detection of Fe3+ by fluorescent chemical sensors. Sens Actuators, B 143:649–654
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghosh, S., Manna, R. & Dey, S. Polyurethane network using 1-naphthylamine embedded epoxy-based polymer: ferric ion selective fluorescent probe. Polym. Bull. 76, 205–213 (2019). https://doi.org/10.1007/s00289-018-2374-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2374-2