Abstract
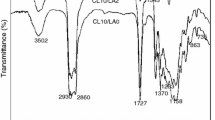
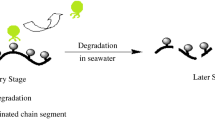
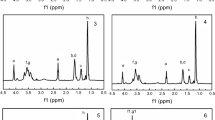
Polyurethane coating materials with different compositions of low surface energy polydimethylsiloxane and degradable poly(l-lactic acid) were synthesized by three major steps. Initially, the hydroxylation-terminated poly(l-lactide)-functionalized graphene (G-g-PLLA) was prepared by ring-opening polymerization of l-lactic acid using the phenol-functionalized graphene (G-f-OH), which was prepared by 1,3-dipolar cycloaddition reaction of graphene and 3,4-dihydroxybenzaldehyde when N-methylglycine and tin octoate were used as initiator and catalyst, respectively. Subsequently, isocyanate-terminated polyurethane prepolymer with polydimethylsiloxane was obtained by condensation polymerization of polydimethylsiloxane and isocyanate-terminated polyurethane prepolymer that was obtained by the condensation polymerization of 4,4′-diphenylmethane diisocyanate and 1,4-butane diol. Finally, the novel polyurethane coating materials were prepared by the condensation polymerization of G-g-PLLA and isocyanate-terminated polyurethane prepolymer with polydimethylsiloxane. These synthesized materials were carefully analyzed with Fourier transform infrared spectroscopy (FTIR), proton nuclear magnetic resonance spectra (1H NMR), field-emission scanning electron microscopy (SEM), and high-resolution transmission (TEM). In addition, the water contact angles were measured. It was found that the surface free energy of the polyurethane coating materials decreased from 52.19 to 11.74 N/m2 with the increase of polydimethylsiloxane content from 0 to 20% and the water contact angle of the polyurethane coating materials increased from 71° to 108°. Moreover, the mechanical property was investigated. The studies also demonstrated that functionalized polyurethane was able to hydrolyze in seawater and the hydrolysis rate decreased as the PDMS content increased. At the same time, simulative ocean hanging plate experiment confirmed that the novel polyurethane coating materials exhibited a good antifouling performance, which indicated that the functionalized polyurethane has a potentiality in marine antifouling coating application.

















Similar content being viewed by others
References
Ma J, Ma C, Yang Y, Xu W, Zhang G (2014) Biodegradable polyurethane carrying antifoulants for inhibition of marine biofouling. Ind Eng Chem Res 53(32):12753–12759
Wang J, Wei J (2016) Hydrogel brushes grafted from stainless steel via surface-initiated atom transfer radical polymerization for marine antifouling. Appl Surf Sci 382:202–216
Chambers LD, Wharton JA, Wood RJK, Walsh FC, Stokes KR (2014) Techniques for the measurement of natural product incorporation into an antifouling coating. Prog Org Coat 77:473–484
Liu Y, Li G (2012) A new method for producing “Lotus Effect” on a biomimetic shark skin. J Colloid Interface Sci 388:235–242
Yee ML, Khiew P, Lim S, Chiu W, Tan Y, Kok Y, Leong C (2017) Enhanced marine antifouling performance of silver-titania nanotube composites from hydrothermal processing. Coll Surf A Physicochem Eng Asp 520:701–711
Wu F, Zhang S, Zhang B, Yang W, Liu Z, Yang M (2016) The effect of the grafted chains on the crystallization of PLLA/PLLA-grafted SiO2 nanocomposite. Colloid Polym Sci 294:801–813
Yang J, Qin Y, Cao J, Yuan M, Liu S, Yuan M (2013) Preparation and characterization of poly(l-lactide)/poly(ε-caprolactone) composite films for food packaging application. Adv Mater Res 750–752:845–848
Ye S, Hong Y, Sakaguchi H (2014) Nonthrombogenic, biodegradable elastomeric polyurethanes with variable sulfobetaine content. ACS Appl Mater Interfaces 6:22796–22797
Song Y, Wang D, Jiang N, Gan Z (2015) Role of PEG segment in stereocomplex crystallization for PLLA/PDLA-b-PEG-b-PDLA blend. ACS Sustain Chem Eng 3:1492–1500
Samarajeewa S, Shrestha R, Li Y, Wooley KL (2012) Degradability of poly(lactic acid)-containing nanoparticles: enzymatic access through a cross-linked shell barrier. J Am Chem Soc 134:1235–1242
Nayebi P, Zaminpaym E (2016) A molecular dynamic simulation study of mechanical properties of graphene–polythiophene composite with Reax force field. Phys Lett A 380:628–633
Jiang H, Zhang Y, Han D, Xi H, Feng J, Chen Q, Hong Z, Sun H (2014) Bioinspired fabrication of superhydrophobic graphene films by two-beam laser interference. Adv Funct Mater 24:4595–4602
Issaadi K, Habi A, Grohens Y, Pillin I (2016) Maleic, anhydride-grafted poly(lactic acid) as a compatibilizer in poly(lactic acid)/graphene oxide nanocomposites. Polym Bull 73:2057–2071
Tang N, Mu L, Qu H, Wang Y, Duan X, Reed MA (2017) Smartphone-enabled colorimetric trinitrotoluene detection using amine-trapped polydimethylsiloxane membranes. ACS Appl Mater Interfaces 9:4445–14452
Jiang Z, Fang S, Wang C, Wang H, Ji C (2016) Durable polyorganosiloxane superhydrophobic films with a hierarchical structure by sol–gel and heat treatment method. Appl Surf Sci 390:993–1001
Planes M, Brand J, Lewandowski S, Remaury S, Sole S, Coz C, Carlotti S, Sebe G (2016) Improvement of the thermal and optical performances of protective polydimethylsiloxane space coatings with cellulose nanocrystal additives. ACS Appl Mater Interfaces 8:28030–28039
Ye S, Majumdar P, Chisholm B, Stafslien S, Chen Z (2010) Antifouling and antimicrobial mechanism of tethered quaternary ammonium salts in a cross-linked poly(dimethylsiloxane) matrix studied using sum frequency generation vibrational spectroscopy. Langmuir 26:16455–16462
Kim Youngwook, Lee Jaemin, Lee Inwon, Lee Sungho, Ko Jongsoo (2013) Skin friction reduction in tubes with hydrophobically structured surfaces. Int J Precis Eng Manuf 14:299–306
Gao Y, Li J, Shum H, Chen H (2016) Drag reduction by bubble-covered surfaces found in PDMS microchannel through depressurization. Langmuir 32:4815–4819
Banerjee S, Mishra A, Singh MM, Maiti B, Ray B, Maiti P (2011) Highly efficient polyurethane ionomer corrosion inhibitor: the effect of chain structure. RSC Adv 1:199–210
Li Y, Gao Jian T, Linliu K, Desper R, Chu B (1992) Multiphase structure of a segmented polyurethane: effects of temperature and annealing. Macromolecules 25:7365–7372
Klinedinst DB, Yilgor E, Yilgor I, Beyer FL, Wilkes GL (2005) Structure–property behavior of segmented polyurethaneurea copolymers based on an ethylene–butylene soft segment. Polymer 46:10191–10201
Yilgor E, Yilgor I, Yurtsever E (2002) Hydrogen bonding and polyurethane morphology. I. Quantum mechanical calculations of hydrogen bond energies and vibrational spectroscopy of model compounds. Polymer 43:6551–6559
Mishra A, Aswal VK, Maiti P (2010) Nanostructure to microstructure self-assembly of aliphatic polyurethanes: the effect on mechanical properties. J Phys Chem B 114:5292–5293
Ou B, Chen M, Huang R, Zhou H (2016) Preparation and application of novel biodegradable polyurethane copolymer. RSC Adv 6:47138–47144
Zhang Y, Qi Y, Zhang Z (2016) Synthesis of PPG–TDI–BDO polyurethane and the influence of hard segment content on its structure and antifouling properties. Prog Org Coat 97:115–121
Cao S, Liu T, Tsang Y, Chen C (2016) Role of hydroxylation modification on the structure and property of reduced graphene oxide/TiO2 hybrids. Appl Surf Sci 382:225–238
Lashgari S, Karrabi M, Ghasemi I, Azizi H, Messori M (2016) Graphene nanoplatelets dispersion in poly(l-lactic acid): preparation method and its influence on electrical, crystallinity and thermomechanical properties. Iran Polym J 25:193–202
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (Grant nos. 51775183, 51275167, 51475161), Hunan Provincial Natural Science Foundation of China (Grant no. 2018JJ2125), Scientific Research Fund of Hunan Provincial Education Department (Grant nos. 15K041, 15A059), Research Innovation Project of Hunan Provincial Graduate Student (Grant no. CX2017B643), Open Research Fund of State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, and the Tribology Science Fund of State Key Laboratory of Tribology, Tsinghua University (Grant no. SKLTKF17B14). So, the affiliation of State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022 China, and State Key Laboratory of Tribology, Tsinghua University, Beijing, 100084 China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ou, B., Chen, M., Guo, Y. et al. Preparation of novel marine antifouling polyurethane coating materials. Polym. Bull. 75, 5143–5162 (2018). https://doi.org/10.1007/s00289-018-2302-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2302-5