Abstract
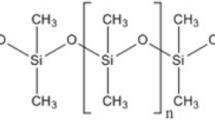
Low-hydrogen-containing polydimethylsiloxane (l-HPDMS) with different hydrogen contents and relative molecular mass was prepared via ring opening equilibrium polymerization, in the fixed-bed reactor which was filled with catalyst particles. The kinetics of D4 ring opening reaction was studied to obtain the kinetic rate model. The physical model was determined by investigating the viscosity of the products at different aspect ratios and reaction temperature. According to the kinetic rate model and physical model, one-dimensional and pseudo-homogeneous ideal plug model was developed to simulate the fixed-bed reactor of l-HPDMS. The concentration of D4 and relative molecular mass curves of products predicted by this model were compared to those experimentally measured in a bench-scale reactor. It can also calculate the bed height of reactor and provide the basis for the design of fixed-bed reactor, for the preparation of different l-HPDMS. Moreover, the effects of liquid hourly space velocity on the relative molecular mass were investigated.














Similar content being viewed by others
Abbreviations
- U :
-
Superficial velocity, m s−1
- C i :
-
Concentration in fluid phase, mol m−3
- η 0 :
-
Effectiveness factor
- z :
-
Reactor longitudinal coordinate, m
- v i :
-
Stoichiometric coefficient
- r M :
-
Reaction rate, mol m−3 s−1
- ρ :
-
Catalyst bed density, kg m−3
- M :
-
Relative molecular mass
- H :
-
Height of catalytic bed, cm
- u 0 :
-
Volume flow at the entrance, mL s−1
- D :
-
Reactor diameter, cm
- ε :
-
Void fraction
- k :
-
Rate constant, s−1
- T :
-
Temperature, °C
- V bed :
-
Bed volume, m3
- V cat :
-
Catalyst volume, m3
- m 0 :
-
Catalyst quality, g
- ρ bed :
-
Catalyst density, kg m−3
- ϕ :
-
Thiele modulus
- Pe :
-
Peclet number
- Da :
-
Damkohler number
- E a :
-
Activation energy, kJ mol−1
- t :
-
Time, min
References
Binet C, Dumont M, Fitremann J (2008) Hydrosilylation of polymethylhydrogenosiloxanes in the presence of functional molecules such as amines, esters or alcohols. Springer, The Netherlands
Lin F, Song MZ, He ZZ, Zhang TY (2008) Synthesis and structural characterization of methacrylic acid/octadecyl acrylate-graft-poly (methylhydrosiloxane) by hydrosilylation. J Appl Polym Sci 107:3773–3780
Wang WZ (2003) Synthesis and characterization of UV-curable polydimethylsiloxane epoxy acrylate. Eur Polym J 39:1117–1123
Byrdn R (1999) U.S. Patent 5858468
Belfield KD, Chinna C, Najjar O (1998) Synthesis of novel polysiloxanes containing charge transporting and second-order nonlinear optical functionalities with atom economical constructs. Macromolecules 31:2918–2924
Safa KD, Tofangdarzadeh S, Hassanpour A (2009) Facile route to synthesis of functionalised poly (methylalkoxy) siloxane under mild and aerobic conditions in the presence of platinum catalysts. J Organomet Chem 694:4107–4115
Tolentino LA, Khanshab AA (2003) U.S. Patent 6534614
Guo DJ, Han HM, Xiao SJ (2007) Surface-hydrophilic and protein-resistant silicone elastomers prepared by hydrosilylation of vinyl poly (ethylene glycol) on hydrosilanes-poly (dimethylsiloxane) surfaces. Colloid Surf A 308:129–135
Saxena A, Markanday M, Sarkar A (2011) A systematic approach to decipher the microstructure of methyl hydrosiloxane copolymers and its impact on their reactivity trends. Macromolecules 44:6480–6487
Yang X, Yao C (2007) Synthesis and comparative properties of poly (dimethylsiloxane) grafted alkyl acrylate. J Appl Polym Sci 106:3600–3604
Hao X, Jeffery JL, Wilkie JS (2010) Functionalised polysiloxanes as injectable in situ curable accommodating intraocular lenses. Biomaterials 31:8153–8163
Yang MH, Lin HT, Lin CC (2003) Synthesis and characterization of phenyl modified PDMS/PHMS copolymers. J Chin Chem Soc-taip 50:51–58
Geisberger G, Lindner T, Baumann F (2008) U.S. Patent 7396894
Cancou TP, Daudet E, Hlary G (2000) Functional polysiloxanes. I. Microstructure of poly(hydrogenmethylsiloxane-co-dimethylsiloxane)s obtained by cationic copolymerization. J Polym Sci Pol Chem 38:826–836
Bukhanko N, Wärnå J, Samikannu A, Mikkola J (2016) Kinetic modeling of gas phase synthesis of ethyl chloride from ethanol and HCl in fixed bed reactor. Chem Eng Sci 142:310–317
Azarpour A, Borhani T, Wan AS, Manan Z, Abdul MM (2017) A generic hybrid model development for process analysis of industrial fixed-bed catalytic reactors. Chem Eng Res Des 117:149–168
Fazlollahnejad M, Taghizadeh M, Eliassi A, Bakeri G (2009) Experimental study and modeling of an adiabatic fixed-bed reactor for methanol dehydration to dimethyl ether. Chin J Chem Eng 17:630–634
Riggs JM (1994) An introduction to numerical methods for chemical engineers. Texas Tech University, Texas
Grzelka A, Chojnowski J, Fortuniak W (2004) Kinetics of the anionic ring opening polymerization of cyclosiloxanes initiated with a superbase. J Ino Org Polym 14:85–99
Morton M, Bostick EE (1964) Anionic polymerization of octamethylcyclotetrasiloxane in tetrahydrofuran solution. J Polym Sci Pol Chem 2:523–538
Lee CL, Johannson OK (1966) Polymerization of cyclosiloxanes. I. Kinetic studies on living polymer-octamethylcyclotetrasiloxane systems. J Polym Sci Pol Chem 4:3013–3026
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ji, C., Li, F. & Yun, Z. Preparation of low-hydrogen-containing polydimethylsiloxane in fixed-bed reactor and establishment of mathematical model. Polym. Bull. 75, 3607–3625 (2018). https://doi.org/10.1007/s00289-017-2231-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-017-2231-8