Abstract
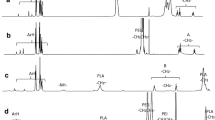
Drug carrier, poly(maleic anhydride-co-vinyl acetate) (MAVA or poly[MA-co-VA]) copolymer, was traditionally synthesized by free radical chain polymerization reaction, in methyl ethyl ketone (MEK) organic media at 80 °C, using benzoyl peroxide (BPO) as the radicalic initiator. The purified copolymer was then modified with a chemotherapeutic agent, doxorubicin hydrochloride (DOX) at 75 °C for 72 h, using N-(3-dimethyl-aminopropyl)-N′-ethylcarbodiimide hydrochloride (EDAC) as the carboxylic acid-activating agent. Structural characterization of the MAVA and the modified MAVA/DOX conjugate was carried out by Fourier transform infrared (FTIR) and nuclear magnetic resonance (1H-NMR and 13C-NMR). Their molecular weights were determined by size-exclusion chromatography (SEC). The spectroscopic and SEC results confirmed that conjugated/modification reaction was successfully carried out. UV spectrophotometric measurements indicated that MAVA/DOX preserved its molecular stability in physiological body fluid, PBS (physiological pH 7.40 at 37 °C). Antiproliferative activities of MAVA/DOX were determined by BrdU cell proliferation ELISA assay using C6 (Rat Brain tumor cells) and HeLa (human uterus carcinoma) cell lines in vitro by comparing with free DOX agent (reference compound). Although MAVA showed low antiproliferative activity, both MAVA/DOX and DOX exhibited greater activity against HeLa and C6. Lactate dehydrogenase (LDH) leakage assay was performed for MAVA/DOX and DOX, which detected a non-toxic effect against C6 even at the highest dose (100 μg/mL). IC50 and IC75 values were also determined using ED50 plus v1.0. Molecular modeling at M06-L/6-31 + G(d,p)//AM1 level showed that the electron density in MAVA/DOX is more localized resulting a higher polarization and thereby a higher dipole moment which shed light on the solubility of MAVA/DOX conjugate.
Graphical abstract













Similar content being viewed by others
References
Thakur VK, Thakur MK, Popescu I (2015) Handbook of polymers for pharmaceutical technologies: processing and applications, vol 2. Scrivener Publishing. doi:10.1002/9781119041412.ch10
Florence AT, Siepmann J (2009) Modern pharmaceutics: basic principles and systems, vol 1, 5th edn. CRC Press, Boca Raton
Bacu E, Chitanu GC, Couture A, Grandclaudon P, Singurel Gh, Carpov A (2002) Potential drug delivery systems from maleic anhydride copolymers and phenothiazine derivatives. Eur Polym J 38:1509–1513
Saad GR, Morsi RE, Mohammady SZ, Elsabee MZ (2008) Dielectric relaxation of monoesters based poly(styrene-co-maleic anhydride) copolymer. J Polym Res 15:115–123. doi:10.1007/s10965-007-9150-6
Atıcı OG, Akar A, Rahimian R (2001) Modification of poly(maleic anhydride-co- styrene) with hydroxyl containing compounds. Turk J Chem 25:259–266
Patel H, Raval DA, Madamwar D, Patel SR (1998) Polymeric prodrug: synthesis, release study and antimicrobial property of poly(styrene-co-maleic anhydride)-bound acriflavine. Angew Makromol Chem 263:25–30
Liu HY, Cao K, Yao Z, Li BG, Hu GH (2007) Variations of the glass-transition temperature in the imidization of poly(styrene-co-maleic anhydride). J Appl Polym Sci 104:2418–2422. doi:10.1002/app.25917
Kumar N, Langer RS, Domb AJ (2002) Polyanhydrides: an overview. Adv Drug Deliv Rev 54:889–910. doi:10.1016/S0169-409X(02)00050-9
Chiellini F, Piras AM, Errico C, Chiellini E (2008) Nanomedicine 3:367–393. doi:10.2217/17435889.3.3.367
Popescu I, Suflet DM, Pelin IM, Chitanu GC (2011) Biomedical applications of maleic anhydride copolymers. Rev Roum Chim 56:173–188
Riabtseva A, Mitina N, Grytsyna I, Boiko N, Garamus VM, Stryhanyuk H, Stoika R, Zaichenko A (2016) Functional micelles formed by branched polymeric surfactants: synthesis, characteristics, and application as nanoreactors and carriers. Eur Polym J 75:406–422. doi:10.1016/j.eurpolymj.2016.01.006
Song F, Li X, Wang Q, Liao L, Zhang C (2014) Nanocomposite hydrogels and their applications in drug delivery and tissue engineering. J Biomed Nanotechnol 10:1–13
Wang L, Kritensen J, Ruffner DE (1998) Delivery of antisense oligonucleotides using HPMA polymer: synthesis of A thiol polymer and its conjugation to water-soluble molecules. Bioconjug Chem 9:749–757
Ulbrich K, Šubr V (2010) Adv Drug Deliver Rev 62:150–166
Kadaji VG, Betageri CV (2011) Water soluble polymers for pharmaceutical applications. Polymers 3:1972–2009. doi:10.3390/polym3041972
Cho K, Wang X, Nie S, Chen ZG, Shin DM (2008) Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res 14:1310–1316. doi:10.1158/1078-0432.CCR-07-1441
Minko T (2005) Soluble polymer conjugates for drug delivery. Drug Discov Today Tech 2:15–20
Li C, Wallace S (2008) Polymer-drug conjugates: recent development in clinical oncology. Adv Drug Deliv Rev 60:886–898
Duncan R (1999) Polymer conjugates for tumour targeting and intracytoplasmic delivery. The EPR effect as a common gateway? Res Focus Rev 2:441–449
Hoste K, Winne KD, Schacht E (2004) Polymeric prodrugs. Int J Pharmaceut 277:119–131
Ringsdorf H (1975) Structure and properties of pharmacologically active polymers. J Polym Sci 51:135–153
Breslow DS (1976) Biologically active synthetic polymers. Pure Appl Chem 46:103–113
Dhal PK, Holmes-Farley SR, Huval CC, Jozefiak TH (2006) Polymers as drugs. Adv Polym Sci 192:9–58
Duncan R (2003) The dawning era of polymer therapeutics. Nat Rev Drug Discov 2:347–360
Greish K, Sawa T, Fang J, Akaika T, Maeda H (2004) SMA-doxorubicin, a new polymeric micellar drug for effective targeting to solid tumors. J Control Release 97:219–230
Maeda H, Ueda M, Morinaga T, Matsumoto T (1985) Conjugation of poly(styrene-co-maleic acid) derivatives to the antitumor protein neocarzinostatin: pronounced improvements in pharmacological properties. J Med Chem 28:455–461
Kobayashi A, Oda T, Maeda H (1988) Protein binding of macromolecular anticancer agent SMANCS: characterization of poly(styrene-co-maleic acid) derivatives as an albumin binding ligand. J Bioact Compat Polym 3:319–333
Ohtsuka N, Konno T, Myauchi Y, Meada H (1987) Anticancer effects of arterial administration of the anticancer agent SMANCS with lipiodol on metastatic lymph nodes. Cancer 59:1560–1565
Maeda H, Matsumoto T, Konno T, Iwai K, Ueda M (1984) Tailor-making of protein drugs by polymer conjugation for tumor targeting: a brief review on Smancs. J Protein Chem 3:181–193
Greish K, Fang J, Inutsuka T, Nagamitsu A, Maeda H (2003) Macromolecular Therapeutics: advantages and prospects with special emphasis on solid tumour targeting. Clin Pharmacokinet 42:1089–1105
Maeda H (1991) SMANCS and polymer-conjugated macromolecular drugs: advantages in cancer chemotherapy. Adv Drug Deliv Rev 6:181–202
DiMarco A, Gaetani M, Scarpinato B (1969) Adriamycin (NSC-123, 127): a new antibiotic with antitumor activity. Cancer Chemother Rep 53:33–37
Bruch M, Mäder D, Bauers F, Loontjens T, Mülhaupt R (2000) Melt modification of poly(styrene-co-maleic anhydride) with alcohols in the presence of 1,3-oxazolines. J Polym Sci 38:1222–1231. doi:10.1002/(SICI)1099-0518(20000415)38:8<1222:AID-POLA5>3.0.CO;2-Z
Ece A, Boris P (2014) A computational insight into acetylcholinesterase inhibitory activity of a new lichen depsidone. J Enzyme Inhib Med Chem. doi:10.3109/14756366.2014.949256
Dewar MJS, Zoebisch EG, Stewart JJP (1985) The development and use of quantum mechanical molecular models. 76. AMI: a new general purpose quantum mechanical molecular model. J Am Chem Soc 107:3902–3909
Zhao Y, Truhlar DG (2006) A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J Chem Phys 125:1–17
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Jacquemin D, Perpete EA, Ciofini I et al (2010) On the performances of the M06 family of density functionals for electronic excitation energies. J Chem Theory Comput 6:2071–2085
Karakus G, Zengin HB, Akin Polat Z, Yenidunya AF, Aydin S (2013) Cytotoxicity of three maleic anhydride copolymers and common solvents used for polymer solvation. Polym Bull 70:1591–1612. doi:10.1007/s00289-012-0860-5
Nguyen V, Yoshida W, Cohen Y (2003) J Appl Polym Sci 87:300–310
Fles D, Vukovic R, Kuzmic AE, Bogdanic G, Pilizota V, Karlovic D, Markus K, Wolsperger K, Vikic-Topic D (2003) Croat Chem Acta 76:69–74
Karakus G, Akin Polat Z, Sahin Yaglioglu A, Karahan M, Yenidunya AF (2013) Synthesis, characterization, and assessment of cytotoxic, antiproliferative, and antiangiogenic effects of a novel procainamide hydrochloride-poly(maleic anhydride-co-styrene) conjugate. J Biomat Sci Polym E 24:1260–1276. doi:10.1080/09205063.2012.750209
Khaon MK (1982) Synthesis of esters. J Org Chem 47:1962–1965
Lundblad RL et al (1984) Modification of carboxyl groups in proteins. Chem Reag Protein Modif 2:105
Chernikova E, Terpugova P, Bui C, Charleux B (2003) Effect of comonomer composition on the controlled free-radical copolymerization of styrene and maleic anhydride by reversible addition-fragmentation chain transfer (RAFT). Polymer 44:4101–4107
Li Y, Richard-Turner S (2010) Free radical copolymerization of methyl substituted stilbenes with maleic anhydride. Eur Polym J 46:821–828
Sundell AM, Luttikhedde HJG (1999) Drug release from ionomer cements based on hydrolyzed poly(vinyl acetate-maleic anhydride). Pure Appl Chem A36:1045–1059
Demirtas I, Sahin A, Ayhan B, Tekin S, Telci I (2009) Antiproliferative effects of the methanolic extracts of sideritis libanotica labill. subsp. linearis. Rec Nat Prod 3:104–109
Demirtas I, Sahin A (2013) Bioactive volatile content of the stem and root of Centaurea carduiformis DC. subsp. carduiformis var. carduiformis. J Chem 2013:125286-1–125286-6. doi:10.1155/2013/125286
Sahin-Yaglıoglu A, Akdulum B, Erenler R, Demirtas I, Telci I, Tekin S (2013) Antiproliferative activity of pentadeca-(8E, 13Z) dien 11-yn-2-one and (E)-1,8-pentadecadiene from Echinacea pallida (Nutt.) Nutt. roots. Med Chem Res. doi:10.1007/s00044-012-0297-2
Frisch MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09, Revision C.01. Gaussian, Inc., Wallingford
Tasdemir IH, Ece A, Kilic E (2012) Experimental and theoretical study on the electrochemical behavior of zofenopril and its voltammetric determination. Curr Pharm Anal 8(4):339–348
(2015) Small-Molecule Drug Discovery Suite 2015-2: QikProp, version 4.4. Schrödinger, LLC, New York
Yoon KJ, Woo JH, Seo YS (2003) Formaldehyde free cross-linking agents based on maleic anhydride copolymers. Fiber Polym 4:182–187
Xiao CM, Tan J, Xue GN (2010) Synthesis and properties of starch-g-poly(maleic anhydride-co-vinyl acetate). Express Polym Lett 4:9–16
Chitanu GC, Popescu I, Carpov A (2006) Synthesis and characterization of maleic anhydride copolymers and their derivatives. 2. New data on the copolymerization of maleic anhydride with vinyl acetate. Rev Roum Chim 51:923–929
Krayukhina MA, Kozybakova SA, Samoilava NA, Babak VG, Karaeva SZ, Yamskov IA (2007) Synthesis and properties of amphiphilic maleic acid copolymers. Russ J Appl Chem 80:1145–1150. doi:10.1134/S1070427207070269
Qiao Z, Xie Y, Chen M, Xu J, Zhu Y, Qian Y (2000) Synthesis of lead sulfide/(polyvinyl acetate) nanocomposites with controllable morphology. Chem Phys Lett 321:504–507
Sunel V, Popa M, Stoican AD, Popa AA, Uglea C (2008) Poly (maleic anhydride-alt-vinyl acetate) conjugate with alkylating agents. Materiale Plastice 45:149–153
Rzayev ZMO (2011) Graft copolymers of maleic anhydride and its isostructural analogues: high performance engineering materials. Int Rev Chem Eng 3:153–215
Pal J, Singh H, Ghosh AK (2004) Modification of LLDPE using esterified styrene maleic anhydride copolymer: study of its properties and environmental degradability. J Appl Polym Sci 92:102–108
Jeong JH, Byoun YS, Ko SB, Lee YS (2001) Chemical modification of poly(styrene-alt-maleic anhydride) with antimicrobial 4-aminobenzoic acid and 4-hydroxybenzoic acid. J Ind Eng Chem 7:310–315
Xiao L, Shimotani H, Ozawa M, Li J, Dragoe N, Saigo K, Kitazawa K (1999) Synthesis of a novel [60]fullerene pearl-necklace polymer, poly(4,4*-carbonylbisphenylene trans-2-[60]fullerenobisacetamide). J Polym Sci Pol Chem 37:3632–3637
John J, Dalal MK, Patel DR, Ram RN (1997) Preparation, properties, and catalytic application of polymer-bound Ru(III) complexes. JMS Pure Appl Chem A34:489–501
Tripp RA, Dluhy RA, Zhao Y (2008) Novel nanostructures for SERS biosensing. Nanotoday (Review) 3:31–37
Kaplan Can H, Doğan AL, Rzaev ZMO, Uner AH, Güner A (2005) Synthesis and antitumor activity of poly(3,4-dihydro-2H-pyran-co-maleic anhydride-co-vinyl acetate). J Appl Polym Sci 96:2352–2359
Balaji R, Nanjundan S (1999) Copolymerization of 3-methoxy-4-methacryloyloxybenzal phenylimine with methyl methacrylate. Eur Polym J 35:1133–1138
Sato M, Ohta R, Handa M, Kasuga K (2002) Thermotropic liquid crystalline polymers containing five-membered heterocyclic groups VIII. Synthesis, and liquid crystalline and photoluminescent properties of semi-rigid polyesters based on a distyrylbenzene analogue of 1,3,4-thiadiazole. Liq Cryst 29:1441–1446
Ghosh S, Banthia AK (2003) An approach to novel polyamidoamine (PAMAM) side chain dendritic polyesterurethane (SCDPEU) block copolymer architectures. Eur Polym J 39:2141–2146
Ferruti P, Ranucci E, Trotta F, Gianasi E, Evagorou EG, Wasil M, Wilson G, Duncan R (1999) Synthesis, characterization and antitumour activity of platinum (II) complexes of novel functionalized poly(amido amine)s. Macromol Chem Phys 200:1644–1654
Karakus G, Akin Polat Z, Yenidunya AF, Zengin HB, Karakus CB (2013) Synthesis, characterization and cytotoxicity of novel modified poly[(maleic anhydride)-co-(vinyl acetate)]/noradrenaline conjugate. Polym Int 62:492–500. doi:10.1002/pi.4341
Zafar F, Sharmin E, Ashraf SM, Ahmad S (2004) Studies on poly(styrene-co-maleic anhydride)-modified polyesteramide-based anticorrosive coatings synthesized from a sustainable resource. J Appl Polym Sci 92:2538–2544
Ak M, Durmus A, Toppare L (2007) Synthesis and characterization of poly(N-(2-(thiophen-3-yl)methylcarbonyloxyethyl) maleimide) and its spectroelectrochemical properties. J Appl Electrochem 37:729–735
Rajput RS, Rupainwar DC, Singh RK, Singh A (2009) Study on characterization and degree of esterification of styrene maleic anhydride by some medicines. Indian J Chem B 48:1597–1600
Wang S, Wang M, Lei Y, Zhang L (1999) “Anchor effect” in poly(styrene maleic anhydride)/TiO2 nanocomposites. J Mater Sci Lett 18:2009–2012
Edwards HGM, Hickmott E, Hughes MA (1997) Vibrational spectroscopic studies of potential amidic extractants for lanthanides and actinides in nuclear waste treatment. Spectrochim Acta A 53:43–53
Coskun M, Seven P (2011) Synthesis, characterization and investigation of dielectric properties of two-armed graft copolymers prepared with methyl methacrylate and styrene onto PVC using atom transfer radical polymerization. React Funct Polym 71:395–401
Hwang S, Lee CH, Ahn IS (2008) Product identification of guaiacol oxidation catalyzed by manganese peroxidase. J Ind Eng Chem 14:487–492
Lee E, Moon BH, Park Y, Hong S, Lee S, Lee Y, Lim Y (2008) Effects of hydroxy and methoxy substituents on NMR data in flavonols. Bull Korean Chem Soc 29:507–510
Nemtoi G, Beldie C, Tircolea C, Popa I, Cretescu I, Humelnicu I, Humelnicu D (2001) Behaviour of the poly(maleic anhydride-co-vinyl acetate) copolymer in aqueous solutions. Eur Polym J 37:729–735
Sun CZ, Lu CT, Zhao YZ, Guo P, Tian JL, Zhang L, Li XK, Lv HF, Dai DD, Li X (2011) Characterization of the doxorubicin-pluronic F68 conjugate micelles and their effect on doxorubicin resistant human erythroleukemic cancer cells. J Nanomed Nanotechnol 2:2–6
Sanmathi CS, Prasannakumar S, Sherigara BS (2004) Terpolymerization of 2-ethoxy ethylmethacrylate, styrene and maleic anhydride: determination of the reactivity ratios. Bull Mater Sci 27:243–249
Ranjbar-Karimi R, Loghmani-Khouzani H (2011) Synthesis of new azines in various reaction conditions and investigation of their cycloaddition reaction. J Iran Chem Soc 8:223–230
Mustata F, Bicu I (2006) P-aminobenzoic acid/cyclohexanon/formaldehyde resins as hardner for epoxy resins. Synthesis and characterization. J Optoelectron Adv M 8:871–875
Barron PF, Hill DJT, O’Donnell JH, O’Sullivan PW (1984) Applications of DEPT experiments to the 13C NMR of copolymers: poly(styrene-co-maleic anhydride) and poly (styrene-co-acrylonitrile). Macromol 17:1967–1972
Sanchez CO, Alvarado F, Bustos CJ, Schott E, Gatica N, Valdebenito K (2007) Synthesis, characterization, thermal stability and fluorescence of hybrid polymers prepared from amino-phenyl esters. Polym Bull 59:19–330. doi:10.1007/S00289-007-0776-7
Ocampo-Fernández M, Herrera AM, Méndez-Bautista T, Garcia-Serrano J (2009) Synthesis and characterization of diethyl-p-vinylbenzyl phosphonate monomer: precursor of ion exchange polymers for fuel cells. Superficies y Vacío 22:6–10
Lai MF, Li J, Liu JJ (2005) Thermal and dynamic mechanical properties of poly(propylene carbonate). J Therm Anal Calorim 82:293–298
Yang X, Zhang X, Liu Z, Ma Y, Huang Y, Chen Y (2008) High-efficiency loading and controlled release of doxorubicin hydrochloride on graphene oxide. J Phys Chem C 112:17554–17558
Acknowledgments
This work was supported by Sciences Research Projects Foundation of Cumhuriyet University (Project No: F258 and F339). Structural characterizations were carried out at Technology Research and Developing Centre, Erciyes University, Kayseri, Turkey. Average molecular weight distribution of MAVA and MAVA/DOX was analyzed by Size-Exclusion Chromatography (SEC) Measurements at Yildiz Technical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of the manuscript solemnly declare that no scientific and/or financial conflicts of interest exist with other people or institutions.
Rights and permissions
About this article
Cite this article
Karakus, G., Ece, A., Yaglioglu, A.S. et al. Synthesis, structural characterization, and antiproliferative/cytotoxic effects of a novel modified poly(maleic anhydride-co-vinyl acetate)/doxorubicin conjugate. Polym. Bull. 74, 2159–2184 (2017). https://doi.org/10.1007/s00289-016-1821-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1821-1