Abstract
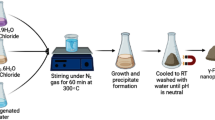
In this work, the silica-coated magnetic field-sensitive iron nanoparticles (Si-γFe2O3) were successfully prepared by sol–gel method and used as the adsorbent in the adsorption of methylene blue. The adsorption capacity was evaluated. Batch adsorption experiments were performed as a function of contact time, initial dye concentration (5–20 mg/L), solution pH (4.0–9.6) and temperature (298–328 K). The structure and the morphology of this material were characterized by the Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM), transmission electron microscopy (TEM) and X-ray diffraction (XRD). The adsorption kinetic studies were achieved using pseudo-first order, and pseudo-second order kinetic models. The adsorption kinetic of methylene blue on Si-γFe2O3 is consistent with the pseudo-second order kinetic model. Thermodynamic parameters such as Gibbs free energy (ΔG°), enthalpy (ΔH°) and entropy (ΔS°) changes were evaluated and the results indicated that adsorption process was endothermic and spontaneous. The equilibrium isotherms for the adsorption of methylene blue on the adsorbent were described by the Freundlich and Langmuir isotherm models.

















Similar content being viewed by others
References
Petcharoena K, Sirivat A (2012) Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater Sci Eng B 177:421–427
Dastjerdi R, Montazer M (2010) A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloid Surf B 79(1):5–18
Ahmada H, Nurunnabia M, Rahmana MM, Kumara K, Tauerb K, Minamic H, Gafurd MA (2014) Magnetically doped multi stimuli-responsive hydrogel microspheres with IPN structure and application in dye removal. Colloid Surf A 459:39–47
Jaquet B, Wei D, Reck B, Reinhold F, Zhang X, Wu H, Morbidelli M (2013) Stabilization of polymer colloid dispersions with pH-sensitive poly-acrylic acid brushes. Colloid Polym Sci 291(7):1659–1667
Serrano-Medinaa A, Cornejo-Bravoa JM, Licea-Claveríeb A (2012) Synthesis of pH and temperature sensitive, core–shell nano/microgels, by one pot, soap-free emulsion polymerization. J Colloid Interface Sci 369(1):82–90
Mornet S, Grasset F, Portier J, Duguet E (2002) Maghemite@silica nanoparticles for biological applications. Eur Cells Mater 3(2):110–113
Debrassia A, Corrêaa FA, Baccarina T, Nedelkob N, Ślawska-Waniewskab A, Sobczakb K, Dłużewskib P, Grenechec JM, Rodriguesa CA (2012) Removal of cationic dyes from aqueous solutions using N-benzyl-O-carboxymethylchitosan magnetic nanoparticles. Chem Eng J 183:284–293
Teja AS, Koh PY (2009) Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog Cryst Growth Ch 55(1–2):22–45
Schütt W, Grüttner C, Häfeli U (1997) Applications of magnetic targeting in diagnosis and therapy—possibilities and limitations. A mini-review. Hybridoma 16(1):109–117
Aroguz AZ, Gulen J, Evers RH (2008) Adsorption of methylene blue from aqueous solution on pyrolyzed petrified sediment. Bioresource Technol 99:1503–1508
Kismir Y, Aroguz AZ (2011) Adsorption characteristics of the hazardous dye Brilliant Green on Saklikent mud. Chem Eng J 172(1):199–206
Tsai WT, Chang CY, Ing CH, Chang CF (2004) Adsorption of acid dyes from aqueous solution on activated beaching earth. J Colloid Interface Sci 275(1):72–78
Xu P, Zeng GM, Huang DL, Feng CL, Hu S, Zhao MH, Lai C, Wei Z, Huang C, Xie GX, Liu ZF (2012) Use of iron oxide nanomaterials in wastewater treatment: a review. Sci Total Environ 424:1–10
Gupta VK, Natyan S (2009) Application of low-cost adsorbents for dye removal: a review. J Environ Manage 90(8):2313–2342
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177(1–3):70–80
Wang Y, Gao C, Yang S (2014) Research of victoria blue B wastewater by coagulation and co-precipitation. Asian J Chem 26(12):3600–3604
Naim MM, El Abd YM (2002) Removal and recovery of dyestuffs from dyeing wastewaters. Sep Purif Rev 31(1):171–228
Gül ÜD, Silah H (2014) Comparison of color removal from reactive dye contaminated water by systems containing fungal biosorbent, active carbon and their mixture. Water Sci Technol 70(7):1168–1174
Bereket G, Aroguz AZ, Ozel MZ (1997) Removal of Pb(II), Cd(II), Cu(II), and Zn(II) from aqueous solutions by adsorption on bentonite. J Colloid Interface Sci 187(2): 338–343
Tsai WT, Chang YM, Lai CW (2005) Adsorption of basic dyes in aqueous solution by clay adsorbent from regenerated bleaching earth. Appl Clay Sci 29(2):149–154
Gulen J, Aroguz AZ, Dalgin D (2005) Adsorption kinetics of azinphosmethyl from aqueous solution onto pyrolyzed Horseshoe Sea crab shell from the Atlantic Ocean. Bioresour Technol 96(10):1169–1174
Dogan M, Abak H, Alkan M (2009) Adsorption of methylene blue onto hazelnut shell: kinetics, mechanism and activation parameters. J Hazard Mater 164(1):172–181
Sismanoglu T, Aroguz AZ (2015) Adsorption kinetics of diazo-dye from aqueous solutions by using natural origin low-cost biosorbents. Desalination Water Treat 54(3):736–743
Kelesoglu S, Kes M, Sutcu L, Polat H (2012) Adsorption of methylene blue from aqueous solution on high lime fly ash: kinetic, equilibrium, and thermodynamic studies. J Disper SciTechnol 33(1):15–23
Aroguz AZ (2006) Kinetics and thermodynamics of adsorption of azinphosmethyl from aqueous solution onto pyrolyzed (at 600 & #xB0;C) ocean peat moss (Sphagnum sp.). J Hazard Mater 135(1–3):100–105
Kishimoto M, Minagawa M, Yanagihara H, Oda T, Ohkochi N, Kita E (2012) Synthesis and magnetic properties of platelet g-Fe2O3 particles for medical applications using hysteresis-loss heating. J Magn Magn Mater 324:1285–1289
Reddy KR, Park W, Sin BC, Noh J, Lee Y (2009) Synthesis of electrically conductive and superparamagnetic monodispersed iron oxide-conjugated polymer composite nanoparticles by in situ chemical oxidative polymerization. J Colloid Interface Sci 335(1):34–39
Reddy KR, Lee KP, Gopalan AI, Kim MS, Showkat AM, Nho YC (2006) Synthesis of metal (Fe or Pd)/alloy (Fe–Pd)-nanoparticles-embedded multiwall carbon nanotube/sulfonated polyaniline composites by γ-irradiation. J Polymer Sci Part A Polym Chem 44:3355–3364
Reddy KR, Lee KP, Gopalan AI (2008) Self-assembly approach for the synthesis of electro-magnetic functionalized Fe3O4/polyaniline nanocomposites: effect of dopant on the properties. Colloids Surf A Physicochem Eng Asp 320:49–56
Reddy KR, Sin BC, Yoo CH, Park W, Ryu KS, Lee JS, Sohn D, Lee Y (2008) A new one-step synthesis method for coating multi-walled carbon nanotubes with cuprous oxide nanoparticles. Scr Mater Scr Mater 58:1010–1013
Showkat AM, Zhang YP, Kim MS, Gopalan AI, Reddy KR, Lee KP. Analysis of heavy metal toxic ions by adsorption onto amino-functionalized ordered (2007) mesoporous silica bull. Korean Chem Soc 28(11):1985–1992
Reddy KR, Lee KP, Gopalan AI, Kang HD (2007) Organosilane modified magnetite nanoparticles/poly (aniline-co-o/m-aminobenzenesulfonic acid) composites: synthesis and characterization. React Funct Polym 67(10):943–954
De Bonis A, Lovaglio T, Galasso A, Santagata A, Teghil R (2015) Iron and iron oxide nanoparticles obtained by ultra-short laser ablation in liquid. Appl Surf Sci 353:433–438
Tural B, Sopacı ŞB, Özkan N, Demir AS, Volkan M (2011) Preparation and characterization of surface modified γ-Fe2O3 (maghemite)–silica nanocomposites used for the purification of benzaldehyde lyase. J Phys Chem Solids 72(8):968–973
Arivoli S, Hema M, Parthasarathy S, Manju N (2010) Adsorption dynamics of methylene blue by acid activated carbon Chem. Pharm Res 2(5):626–641
Sanghi Rashmi, Verma Preeti (2013) Decolorisation of aqueous dye solutions by low-cost adsorbents: a review. Color Technol 129:85–108
Lagergren S (1898) Zur theorie der sogenannten adsorption gelöester stoffe. K Sven Vetensk Handl 24(4):1–39
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Mathews AP, Weber WJ (1976) Effects of external mass transfer and inter-particle diffusion on adsorption. AIChE Symp Ser 73:91–98
Coskun R, Delibas A (2012) Removal of methylene blue from aqueous solutions by poly(2-acrylamido-2-methylpropane sulfonic acid-co-itaconic acid) hydrogels. Polym Bull 68:1889–1903
Vijayakumar G, Tamilarasan R, Dharmendirakumar M (2012) Adsorption, kinetic, equilibrium and thermodynamic studies on the removal of basic dye rhodamine-B from aqueous solution by the use of natural adsorbent perlite. J Mater Environ Sci 3(1):157–170
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and Platinum. J Am Chem Soc 40(9):1361–1403
Freundlich HMF (1906) Über die adsorption in lösungen. Z Phys Chem 57A:385–470
Aksu Z (2002) Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of nickel (II) ions onto Chlorella vulgaris. Process Biochem 38:89–99
Acknowledgments
This work was supported by Scientific Research Projects Coordination Unit of Istanbul University. Project number: BAP-5361. Financial support of Istanbul University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with ethical standards
We are submitting our manuscript entitled “The synthesis of silica-coated magnetic nanoparticles and using as adsorbent” for publication in Polymer Bulletin. Our manuscript has not been submitted to more than one journal for simultaneous consideration. As a result of above ethical statements, this work has been carried out with ethical standards.
Conflict of interest
This work was supported by the Research Fund of the Istanbul University. Project number: BAP-5361. We have already reported this research grants from Fund of İstanbul University at the acknowledgement section in the manuscript. Financial support of Istanbul University is gratefully acknowledged. No honoraria for speaking at symposia. There is no financial support for attending any symposia. There is no financial support for educational programs. No employment or consultation for this work. There is no support from a project sponsor. There is no position on advisory board or board of directors or other type of management relationships. There are no multiple affiliations; no financial relationships, for example equity ownership or investment interest. There are no intellectual property rights (e.g., patents, copyrights and royalties from such rights). No holdings of spouse and/or children that may have financial interest in the work. In addition, there are no financial interests and compensation (non-financial interests). There are no personal relationships or competing interests directly or indirectly tied to this research, or professional interests or personal beliefs that may influence the research.
Rights and permissions
About this article
Cite this article
Aroguz, A.Z., Sayılı, G. The synthesis of silica-coated magnetic nanoparticles and using as adsorbent. Polym. Bull. 73, 2353–2372 (2016). https://doi.org/10.1007/s00289-016-1612-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1612-8