Abstract
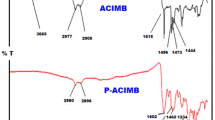
Several poly(thiophene) derivatives containing triphenylamine (TPA) as electron-donor were synthesized by chemical homopolymerization in CHCl3 media using FeCl3 as oxidizing agent. Monomers containing TPA bonded by imine groups to terminal thiophene, bithiophene, terthiophene units allows polymerization to be performed in conditions similar to thiophene. TPA units are regularly spaced in 2, 4, 6 and 8 thiophenyl units in the main chain. TPA electron-donor effect on the polymers chains, as compared to poly(thiophene) was studied. Polymers, labeled as poly(TPA-Th), poly(TPA-biTh) and poly(TPA-Terth) were characterized by 1H-NMR, FT-IR and UV–visible spectroscopy, elemental analysis, thermal stability (TGA) intrinsic viscosity, differential scanning calorimetry (DSC) and electrochemically using cyclic voltammetry (CV). The characterizations are consistent with the proposed structures. The polymers exhibited different optical absorption. They exhibited low intrinsic viscosity, a different effective conjugation and high thermal stability. Moreover, the polymers displayed two redox processes with a redox potential lower than that of poly(thiophene). Highest Occupied Molecular Orbital (HOMO), Lowest Unoccupied Molecular Orbital (LUMO) and optical band gap (E g) were measured and the obtained values were compared with those of poly(thiophene). The effect of the presence of TPA units in the thiophenyl chains on HOMO, LUMO, band gap, redox potential and on TGA is reported. To complete the series, HOMO/LUMO levels and band gap of a polymer containing TPA with 8 thiophenyl units in the chain were determined using theoretical calculations. The results proved that, with respect to poly(thiophene), it is possible to decrease HOMO and LUMO without changing the band gap, projecting itself as a potential polymer to be studied in organic photocells.








Similar content being viewed by others
References
Friend RH, Gymer RW, Holmes AB, Burroughes JH, Marks RN, Taliani C, Bradley DDC, Dos Santos DA (1999) Electroluminescence in conjugated polymers. Nature (London) 397:121–128
Bernius MT, Inbasekaran M, Brien JO (2000) Progress with light-emitting polymers. Adv Mater 12:1737–1750
Wang X, Li H, Liu P (2014) Well-defined aniline-triphenylamine copolymer nanotubes: preparation, photoluminescent, and electrochemical properties. Electrochim Acta 25:630–636
Li Z, Chen Y, Du Y, Wang X, Yang P, Zheng J (2012) Triphenylamine-functionalized graphene decorated with Pt nanoparticles and its application in photocatalytic hydrogen production. Int J Hydrogen Energy 37:4880–4888
Mikroyannidis JA, Stylianakis MM, Suresh P, Balraju P, Sharma GD (2009) Low band gap vinylene compounds with triphenylamine and benzothiadiazole segments for use in photovoltaic cells. Org Electron 10:1320–1333
Roquet S, Cravino A, Leriche P, Aleveque O, Frere P, Roncali J (2006) Triphenylamine–thienylenevinylene hybrid systems with internal charge transfer as donor materials for heterojunction solar cells. J Am Chem Soc 128:3459–3466
Ma C, Zhang B, Liang Z, Xie P, Wang X, Zhang B, Cao Y, Jiang X, Zhang ZA (2002) Novel n-type red luminescent material for organic light-emitting diodes. J Mater Chem 12:1671–1675
Zhu L, Yang HB, Zhong C, Li CM (2014) Rational design of triphenylamine dyes for highly efficient p-type dye sensitized solar cells. Dyes Pigment 105:97–104
Tarsang R, Promarak V, Sudyoadsuk T, Namuangruk S, Jungsuttiwong S (2014) Tuning the electron donating ability in the triphenylamine based D–π–A architecture for highly efficient dye-sensitized solar cells. J Photochem Photobiol A 273:8–16
Liu X, Cao Z, Huang H, Liu X, Tan Y, Chen H, Pei Y, Tan S (2014) Novel D–D–π–A organic dyes based on triphenylamine and indole-derivatives for high performance dye-sensitized solar cells. J Power Sources 248:400–406
Meng F, Liu C, Hua J, Cao Y, Chen K, Tian H (2003) Novel linear and tri-branched copolymers based on triphenylamine for non-doping emitting materials. Eur Polym J 39:1325–1331
Qu J, Kawasaki R, Shiotsuki M, Sanda F, Masuda T (2006) Synthesis and properties of polyacetylenes carrying N-phenylcarbazole and triphenylamine moieties. Polymer 47:6551–6559
He Q, Huang H, Lin H, Yang J, Bai F (2003) Synthesis and Characterization of a novel hyperbranched oligomer with 1,3,5-trisphenylbenzene as cores. Synth Met 135:165–166
Palewicz M, Iwan A, Doskocz J, Strek W, Sek D, Kaczmarczyk B, Mazurek B (2011) Optical and structural study of thin film of polyazomethine with triphenylamine unit prepared via spin-coating method. Polym Bull 66:65–76
Niu H, Huang Y, Bai X, Li X, Zhang G (2004) Study on crystallization, thermal stability and hole transport properties of conjugated polyazomethine materials containing 4,4′-bisamine-triphenylamine. Mater Chem Phys 86:33–37
Niu HJ, Huang YD, Bai X, Li X (2004) Novel poly-Schiff bases containing 4,4′-diamino-triphenylamine as hole transport material for organic electronic device. Mater Lett 58:2979–2983
Sek D, Iwan A, Jarzabek B, Kaczmarczyk B, Kasperczyk J, Mazurak Z, Domanski M, Karon K, Lapkowski M (2008) Hole transport triphenylamine–azomethine conjugated system: synthesis and optical. Photoluminescence Electrochem Prop Macromol 41:6653–6663
Yang C-J, Jenekhe SA (1995) Conjugated aromatic polyimines, and properties of new aromatic polyazomethines. Macromolecules 28:1180–1196
Banerjee S, Saxena C, Gutch PK, Gupta DC (1996) Poly-schiff bases II. Synthesis and characterization of polyetherketoimines. Eur Polym J 32:661–664
Diaz FR, Moreno J, Tagle LH, East GA (1999) Synthesis, characterization and electrical properties of polyimines derived from selenophene. Synth Met 100:187–193
Hathoot AA et al (2000) Electro-oxidative polymerization of Schiff-base of 1,8-diaminonaphthaline and 3-acetylthiophene. I. Preparation and study the redox behaviour of the resulting polymer. Eur Polym J 36:1063–1071
Diab AS, Hathoot AA, Abdel-Azzem M, Merz A (2000) Preparation of a novel conducting polymer by electropolymerization of thiophenylidine 8-naphthylamine Schiff-base. Eur Polym J 36:1959–1965
Simionescu CI, Grigoras M, Cianga I, Diaconu I, Farcas A (1994) Chemical synthesis of some Schiff base type polymers containing pyrrole units. Polym Bull 32:257–264
Sánchez CO, Bèrnede JC, Cattin L, Makha M, Gatica N (2014) Schiff base polymer based on triphenylamine moieties in the main chain. Characterization and studies in solar cells. Thin Solid Films 562:495–500
Frisch MJ, Frisch J, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR et al (2004) Gaussian 03, Revision c.02. Gaussian Inc., Wallingford
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A Gen Phys 38:3098–3100
Lee C, Yang W, Parr RG (1988) Development of The Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B Condens Matter 37:785–789
Rassolov VA, Ratner MA, Pople JA, Redfern PC, Curtiss LA (2001) 6-31G* basis set for third-row atoms. J Comput Chem 22:976–984
Liou GS, Hsiao SH, Huang NK, Yang YL et al (2006) Synthesis, photophysical, and electrochromic characterization of wholly aromatic polyamide blue-light-emitting materials. Macromolecules 39:5337–5346
Roncali J (1997) Synthetic principles for bandgap control in linear π-conjugated sistems. Chem Rev 97:173–206
Roncali J, Garnier F, Lemaire M, Garreau R (1986) Poly mono, bi- and trithiophene: effect of oligomer chain length on the polymer properties. Synth Met 15:323–331
Ribeiro AS, Gazotti WA, Nogueira VC, Machado DA, Dos santos Filho PF, De Paoli MA (2004) Poly(3-alkyl)thiophene derivatives as promising materials assembling an electrochromic device. J Chil Chem Soc 49:197–204
Csaba V, Lukkari J, Kankare J (1994) Electrochemically polymerized terthiophene derivatives carrying aromatic substituents. Macromolecules 27:3322–3329
Roncali J (1992) Conjugated poly(thiophenes): synthesis, functionalization and applications. Chem Rev 92:711–738
Zamora P, Diaz FR, Valle MD, Louarn G, Cattin L, Bernède JC (2013) Redox study of polyaniline derivatives for potential use in photovoltaic devices. Int J Sci 2:1–15
Bavastrello V, Carrara S (2004) Optical and electrochemical properties of poly(o-toluidine) multiwalled carbon nanotubes composite Langmuir–Schaefer films. Langmuir 20:969–973
Brovelli F, Bernède JC, Valle MA, Díaz FR, Berredjem Y (2007) Electrochemical and optical studies of 1,4-diaminoanthraquinone for solar cell applications. Polym Bull 58:521–527
Link S, Richter T, Yurchenko O, Heinze J, Ludwigs S (2010) Electrochemical behavior of electropolymerized and chemically synthesized hyperbranched polythiophenes. J Phys Chem B 114:10703–10708
Ng SC, Miao P (1999) Electrochemical synthesis and characterization studies of poly[3,3′-dialkylsulfanyl-2,2′-bithiophene] films. Macromolecules 32:5313–5320
Wei Y, Chan CC, Tian J, Jang GW, Hsueh KF (1991) Electrochemical polymerization of thiophenes in the mechanism of the polymerization. Chem Mater 3:888–897
Fichou D, Horowitz G, Xu B, Gamier F (1990) Stoichiometric control of the successive generation of the radical cation and dication of extended–conjugated oligothiophenes: a quantitative model for doped polythiophene. Synth Met 39:243–259
Acknowledgments
The authors thank Fondecyt financial support through project 1120055.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sánchez, C.O., Schott, E., Zárate, X. et al. Effect of triphenylamine as electron-donor evenly spaced in 2, 4, 6 and 8 thiophene units of the main chain: synthesis and properties. Polym. Bull. 72, 897–913 (2015). https://doi.org/10.1007/s00289-015-1313-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1313-8